-
PDF
- Split View
-
Views
-
Cite
Cite
John A. Branda, Tai-Yuan David Lin, Eric S. Rosenberg, Elkan F. Halpern, Mary Jane Ferraro, A Rational Approach to the Stool Ova and Parasite Examination, Clinical Infectious Diseases, Volume 42, Issue 7, 1 April 2006, Pages 972–978, https://doi.org/10.1086/500937
Close - Share Icon Share
Abstract
Background. Examination of multiple stool specimens per patient to rule out parasitic infection continues to be recommended in the literature. Attractive alternatives have been proposed, such as examination of a single specimen, but data to support their use have been inconclusive.
Methods. We reviewed the results of comprehensive stool ova and parasite examinations performed during a 1-year period to determine the incremental value of examining >1 specimen. Next, we implemented rejection criteria, allowing analysis of only a single specimen in most cases, and studied the impact of the change by reviewing data from a subsequent year.
Results. Prior to implementation of rejection criteria, 91% of parasites were detected in the first specimen submitted, although many clinical evaluations (72%) involved the submission of only 1 stool specimen. When at least 3 specimens were submitted, the sensitivity of examining the first in the series was 72%. Even the latter sensitivity provides negative predictive values of ∼98%, ∼97%, ∼95%, or ∼93% when the prevalence of parasites among those tested is 5%, 10%, 15%, or 20%, respectively. Examination of additional specimens after examination of the first specimen that yielded a positive finding revealed previously undetected parasites in only 10% of cases. After the application of rejection criteria, the parasite detection rate did not change significantly.
Conclusions. Comprehensive examination of a single stool specimen is sufficient for most patients, when the prevalence of infection among the tested population is up to 20%. Rational use of the stool ova and parasite examination relies on communication between clinician and laboratory, and retention of deferred specimens in case examination of additional specimens is clinically warranted.
For decades, there has been a continuing debate in the scientific literature about whether the reliable detection of intestinal parasites requires examination of multiple stool specimens per patient. Many advocate the separate examination of multiple stool specimens [1–4], but others have proposed pooling multiple specimens for analysis by a single examination [5–7] or the application of an algorithm requiring a negative test result and persistence of symptoms before analysis of a second (or third) specimen [8–10]. Although the debate is acknowledged in authoritative microbiology manuals and published guidelines, these have so far remained conservative, and continue to favor the separate examination of multiple specimens collected on alternate or successive days [11, 12]. The same is true for major clinical textbooks [13, 14], and so it is still contrary to the conventional wisdom for clinicians and laboratory directors to choose alternative approaches. In the interest of cost containment and good resource management, however, it is important to give the alternatives serious consideration, as stool ova and parasite (O&P) examinations are particularly labor intensive and require a high level of technical expertise. Therefore, as part of a laboratory service improvement initiative, we performed a retrospective analysis of stool O&P examination results at our hospital to determine the incremental value of examining >1 specimen per patient. On the basis of our findings, we implemented new rejection criteria and studied the impact of that change on our diagnostic yield.
Methods
In the study's first phase, the results of all stool O&P examinations performed during calendar year 2001 at Massachusetts General Hospital (Boston) were reviewed retrospectively. All specimens received during this time period had been processed without the application of rejection criteria, regardless of the number of specimens submitted per patient. The only exception was that stool specimens collected from patients who had been hospitalized for >3 days at the time of collection were examined only after consultation between the requesting physician and the laboratory.
Multiple specimens from a single patient were considered to be related to the same clinical evaluation if the additional specimens were collected within 14 days after the first. Specimens collected >14 days after the first were considered to be part of a separate clinical evaluation, because of the likelihood that they were collected during a separate episode of gastrointestinal illness or potential exposure. A “series” of stool specimens is defined as ⩾1 specimens collected within a 14-day period from the same patient.
Most stool specimens obtained for stool O&P examination during the reviewed time period consisted of a fresh portion and a portion preserved in sodium acetate—acetic acid—formalin (Para-Pak SAF/Clean; Meridian Bioscience). After straining and centrifugation, the preserved portion was used to prepare permanently stained smears (chlorazol black E stain [15] and modified Kinyoun acid-fast stain [16]) and a slide for direct detection of Cryptosporidium species and Giardia species by immunofluorescence (MeriFluor Cryptosporidium/Giardia, Meridian Bioscience). Concentration wet mounts (saline and D'Antoni's iodine) [11] were then prepared with the formalin-ethyl acetate method [11, 17]. The fresh portion of the specimen was directly examined with a second chlorazol black E–stained slide.
In instances where transport of the specimen to the laboratory within a few hours of collection was possible, fresh stool specimens were sometimes accepted without an accompanying preserved portion. Most specimens were obtained from inpatients and represent a minority of specimens overall. The unpreserved stool specimen was directly examined using wet mounts (saline and D'Antoni's iodine) [11], permanently stained smears (chlorazol black E and modified Kinyoun acid-fast stains), and direct immunofluorescence for Cryptosporidium species and Giardia species. Concentration wet mounts (saline and D'Antoni's iodine) were then prepared with the formalin-ethyl acetate method.
For the purpose of this study, only parasites known to cause intestinal disease were considered, and nonpathogenic organisms (such as Entamoeba coli) were not included in the analysis. Blastocystis hominis was also excluded from consideration, because its pathogenicity is uncertain [18]. Statistical significance was determined using the χ2 test. Differences between proportions were considered to be significant if P ⩽ .05.
After the phase-1 data were analyzed, new rejection criteria were implemented, so that only the first in a series of stool specimens submitted for stool O&P examination was routinely evaluated. Subsequent specimens obtained from the same patient (if collected within 14 days after the first) were not automatically examined, but instead were accessioned, preserved with sodium acetate—acetic acid—formalin, and retained in the laboratory for a period of 30 days. The accompanying stool O&P examination request was cancelled and credited, and a comment was included in the laboratory report explaining that the specimen would only be examined if a health care provider specifically contacted the laboratory. Typically, a second or third stool specimen was examined if ⩾1 of the following conditions was met: the patient remained symptomatic after analysis of the first specimen was complete, the patient was known to have had contact with an infected individual, the patient had recently been in an area where parasites known to cause intestinal disease are endemic, or the patient was immunocompromised.
To evaluate the effects of these rejection criteria, which were applied beginning in July 2003, the results of all stool O&P examinations performed during 1 October 2003–30 September 2004 (the “postimplementation period”) were reviewed. This retrospective analysis represents the second phase of the study. Note that the same methodology was applied to all stool O&P examinations performed during the postimplementation period as was described for examinations performed during calendar year 2001 (the “preimplementation period”). The methods for statistical analysis of the phase-2 data were the same as those described for phase 1.
Results
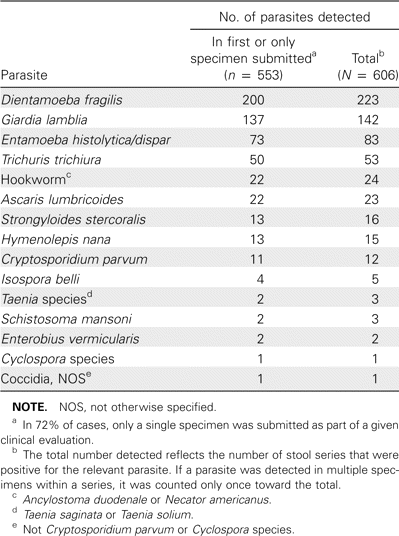
During the preimplementation period, 3684 series of stool specimens were examined. Despite the fact that rejection criteria were not yet being used during the preimplementation period, the majority of series (2656 [72%] of 3684) consisted of only a single stool specimen. Of 3684 total series, 467 were positive (defined as the detection of a pathogenic parasite in at least 1 specimen in the series), and a total of 606 pathogenic parasites were detected. The total number of parasites detected exceeds the total number of positive series, because >1 species of parasite was detected in some series. Most parasites (553 [91%] of 606) were detected either in a single specimen (when only 1 was submitted) or in the first of a series of multiple specimens (table 1). Examination of a second specimen, when submitted, allowed detection of 36 parasites (in 6% of 606 specimens) that had not been detected in the initial specimen of a series. Examination of a third specimen, when obtained, uncovered the remaining parasites (17 [3%] of 606) not detected in either the first or second specimen.
Pathogenic parasites detected by stool ova and parasite examination during the preimplementation period.
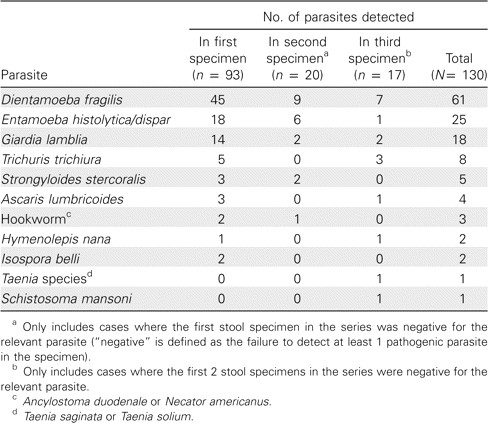
To determine the value of analyzing >1 specimen, the positivity rate (defined as the ratio of positive series to total series) for single-specimen submissions was compared with that for multiple-specimen series. Evaluations limited to a single specimen yielded a positivity rate of 11.9% (317 of 2656 single-specimen submissions), which is not significantly different from that of 2-specimen series (11.6% [53 of 456]; P = .85). Series consisting of at least 3 stool specimens, however, yielded a significantly higher positivity rate (17.0% [97 of 572]) than did single-specimen submissions (P = .001) or 2-specimen series (P = .02). To investigate this further, all 97 positive series consisting of ⩾3 stool specimens were analyzed (table 2). Note that only 2 of the 97 series consisted of >3 specimens; the remainder consisted of 3 specimens per series. Of the parasites detected, 93 (72%) of 130 were discovered in the first specimen, whereas 20 (15%) of 130 were initially detected in the second specimen, and 17 (13%) of 130 were identified in the third after the first 2 specimens in the series were negative (negative is defined as the failure to detect at least 1 pathogenic parasite). Thus, 37 (28%) of the 130 parasites ultimately identified would have been missed if these work-ups had been limited to examination of a single stool specimen. In both of the 2 series consisting of >3 specimens, the additional specimens did not reveal a parasite not already discovered previously in the series.
Pathogenic parasites detected in series consisting of ⩾3 stool specimens for ova and parasite examination during the preimplementation period.
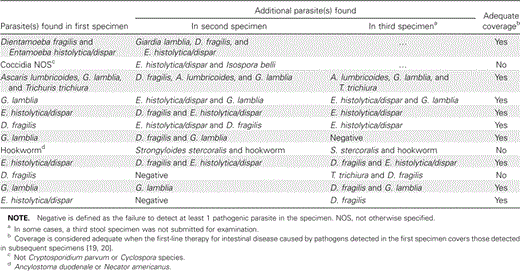
We also considered the benefit of examining subsequent specimens when the first in a series was positive. All series fitting this description from the preimplementation period were analyzed. Overall, examination of additional specimens provided new information (i.e., a pathogenic parasite was detected that had not been detected in the first specimen) in 12 (10%) of 117 cases. Most of the additional parasites (9 of 12) were detected in the second specimen of the series, and 3 of 12 were detected only in the third specimen. These 12 cases were reviewed to determine whether the additional parasites detected would have been susceptible to the recommended drug therapy for those identified in the first stool specimen (table 3). In 9 (75%) of 12 cases, the drug(s) of choice for the parasites detected in the first stool specimen could be expected to be active against those revealed in subsequent specimens in the series [19, 20].
Additional parasites detected by stool ova and parasite examination in a second or third specimen when the first was positive during the preimplementation period.
Phase 2 of the study was designed to investigate whether the implementation of rejection criteria in July 2003 affected our ability to detect pathogenic intestinal parasites. First, we compared the positivity rate for series of stool specimens examined before and after the change. The overall positivity rate for the preimplementation period was 12.7% (467 of 3684 series). The positivity rate for the postimplementation period was 11.8% (457 of 3881 series), which is not significantly different from the rate prior to the change (P = .23). Dientamoeba fragilis was detected in significantly fewer series during the postimplementation period than during the preimplementation period (131 of 3881 series vs. 223 of 3684 series; P < .001). A reverse trend was seen with Giardia lamblia (detected in 185 of 3881 postimplementation series and 142 of 3684 preimplementation series; P = .05) and Cryptosporidium parvum (detected in 31 of 3881 postimplementation series and 12 of 3684 preimplementation series; P = .006). For all other pathogenic parasites identified, the difference in positivity rate between the preimplementation and postimplementation periods was not significant (P > .05; data not shown).
Discussion
Although the need for serial stool O&P examinations has been studied on several occasions, the findings have been inconsistent and sometimes difficult to interpret, leading to a continued lack of consensus on the issue. The root of the problem may be the wide variation in methodology applied to these investigations. As Morris et al. [8] have noted, many reports derive their data from the screening of high-prevalence populations among whom the majority of individuals tested are asymptomatic [1, 2, 21–28]. These studies are not directly relevant to patient care in settings where symptomatic patients are frequently seen and tested, and therefore, their findings may not be generalizable. Similarly, the test protocols employed in early investigations often lacked ⩾1 techniques that today are widely used and considered to be important for the sensitive detection of intestinal parasites. For example, some lacked a concentration technique [29, 30], or did not include permanently stained smears [5, 29–31]. Although many of these studies report that the sensitivity of a single stool examination is inadequate, it is problematic to apply these findings to the current situation. In addition, many studies did not include a parasite-specific antigen test (fecal immunoassay) in combination with the permanently stained slides and concentration wet mount [1, 2, 4, 8, 10, 22, 32], and yet antigen tests are now commonly used in clinical laboratories [33] and are sometimes included as part of the routine evaluation [34], as they are in our laboratory. Given the high sensitivity of these assays [35], their absence must be recognized and considered when interpreting the findings of these studies. Finally, some investigations have described small numbers of patients [26, 27, 30], have focused only on a single species of parasite [5, 21, 28, 30], or have used animal models rather than human subjects [29].
Using a comprehensive and multifaceted stool O&P examination, we found that 91% of parasites detected during the preimplementation period were discovered either in a single specimen or in the first of a series of multiple specimens. One important consideration, however, is that the majority of stool O&P examination series processed during this period consisted of only a single stool specimen. It appears that, even prior to the implementation of rejection criteria, our clinicians only occasionally requested multiple stool specimens per patient (although the preponderance of single-specimen evaluations could also be explained if patients frequently failed to provide subsequent specimens when requested). Realizing that this unforeseen trend could lead us to underestimate the total number of parasites detected in the preimplementation period and, thereby, to overestimate the sensitivity of single-specimen evaluations, we compared the positivity rate for series consisting of 1, 2, and ⩾3 specimens. The positivity rate for ⩾3-specimen series significantly exceeded that for single specimen submissions and for 2-specimen series, suggesting that evaluating ⩾3 specimens is, indeed, more sensitive.
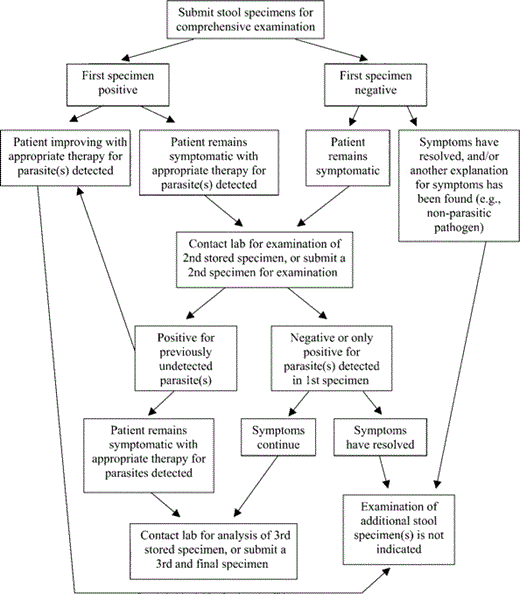
When we focused only on series where ⩾3 specimens were examined, we found that examination of the first stool specimen provided 72% sensitivity, compared with the “gold standard” of ⩾3 separate examinations on separate specimens. Although this level of sensitivity might be unacceptable in a setting where the prevalence of parasites is high, it affords excellent negative predictive value when the prevalence of parasites (among those clinically tested for stool O&P) is moderate or low. For example, the negative predictive value, when the prevalence is 5%, 10%, 15%, or 20%, would be ∼98%, ∼97%, ∼95%, or ∼93%, respectively, assuming 100% specificity [36]. At hospitals and testing centers in the United States, the prevalence of parasites (defined as the proportion of tested patients with at least 1 positive stool specimen) is generally <20% and is frequently <5% [33, 37]. In most instances, therefore, comprehensive examination of a single stool specimen is adequate to rule out parasitic infection in the majority of patients who require stool O&P testing (figure 1). Naturally, if an individual patient has clinical or epidemiological features that confer a higher pretest probability of parasitic infection than the average patient, this will reduce the negative predictive value of a single stool examination for that individual. For such patients, a single negative finding in a stool examination may not allow the clinician to rule out parasitic infection with confidence, and examination of further specimens is justified. Similarly, if significantly less-comprehensive (and presumably less-sensitive) methods than ours are applied to the routine stool analysis, examination of multiple specimens may be justifiable to achieve adequate sensitivity. Although it is acknowledged that our routine procedures are more extensive than those applied in many laboratories, we believe that it is preferable to strengthen the routine analysis rather than to attempt to enhance its sensitivity by repeating it serially. Finally, although our patient population is diverse and includes groups such as pediatric and immunocompromised patients, a stratified analysis by distinct patient subpopulation was not performed and, therefore, it is unknown whether our results are generalizable across such categories.
Algorithm for stool ova and parasite testing in symptomatic patients. In settings of low-to-moderate parasite prevalence (where ⩽20% of tested patients have at least 1 positive finding from a stool specimen), examination of a single stool specimen is generally sufficient. One stool specimen should be analyzed by a comprehensive examination including (at a minimum) a permanently stained smear, a concentration wet mount, and fecal immunoassay(s) directed against Giardia and Cryptosporidium species. A direct wet mount should be included if the specimen is fresh (unpreserved). If additional specimens are submitted (no more than 3 in total) they should be preserved, accessioned, and stored by the laboratory, but processing and examination should be deferred until examination of the first specimen is complete.
The same principles apply when a single specimen is positive. Our analysis of data from the preimplementation period shows that, if a second or third stool specimen is examined after the first is found to be positive, the extra specimens fail to provide additional useful information in 90% of cases. In cases where analysis of additional stool specimens did provide new information, the parasites detected in subsequent specimens were usually susceptible to the primary therapy for those identified in the first specimen. This suggests that additional stool specimens could (for the most part) be safely omitted, assuming that therapy is initiated on the basis of the findings of the first positive specimen, but the small number of such cases in our study is inadequate to prove this. We conclude that a single positive specimen is usually sufficient to determine the appropriate treatment when the examination is comprehensive, but analysis of additional specimens is appropriate in the event of treatment failure or if clinical suspicion of coinfection is high for other reasons.
In the second phase of our study, we reviewed the results of all stool O&P examinations performed during a 1-year period subsequent to the application of rejection criteria. Despite the fact that multiple-specimen series had been reduced from 1028 instances in the preimplementation period to just 73 instances in the postimplementation period (data not shown), the parasite detection rate (i.e., the positivity rate) did not change significantly. This suggests that the implementation of rejection criteria did not adversely affect our ability to detect parasites, assuming that the patient population whose samples were tested did not change substantially from the preimplementation period to the postimplementation period. A comparison of the detection rate for individual species between the 2 time periods shows that the rates are comparable for most parasites. G. lamblia and C. parvum were detected significantly more frequently during the postimplementation period than in the preimplementation period, but D. fragilis was detected significantly more frequently during the preimplementation period than in the postimplementation period. Because these trends run in both directions, it is likely that the differences are attributable to true changes in parasite prevalence rather than to changes in detection sensitivity related to our rejection criteria.
In summary, reflexive submission of multiple stool specimens for O&P examination can be avoided without sacrificing the predictive value of a negative result if the initial analysis is comprehensive and targets organisms that are prevalent in the region. Our data show that comprehensive analysis of a single stool specimen provides adequate sensitivity for most clinical situations in settings where the parasite prevalence among those tested for stool O&P is as high as 20%. If the first stool specimen is negative, a second or third specimen should be examined if symptoms persist and/or there is high clinical suspicion of a parasitic infection. If the first specimen is positive, additional specimens are usually noncontributory and should be examined only in the event of treatment failure or when there is another reason to suspect coinfection. In this way, the testing algorithm is customized for the patient through the application of clinical judgment, and unnecessary testing can be avoided. Preservation and storage of deferred specimens within the laboratory will optimize turnaround time and reduce physician and patient inconvenience if multiple stool examinations are ultimately deemed necessary.
Acknowledgments
We are grateful to Luz Stella Sierra and Mary Anne Waldron for expert laboratory services; Drs. James W. Smith and Robert C. Moellering, Jr., for reviewing the manuscript; and Paula Spagnuolo for secretarial assistance.
Potential conflicts of interest. All authors: no conflicts.





Comments