-
PDF
- Split View
-
Views
-
Cite
Cite
Raha Parvizi Omran, Bernardo Ramírez-Zavala, Walters Aji Tebung, Shuangyan Yao, Jinrong Feng, Chris Law, Vanessa Dumeaux, Joachim Morschhäuser, Malcolm Whiteway, The zinc cluster transcription factor Rha1 is a positive filamentation regulator in Candida albicans, Genetics, Volume 220, Issue 1, January 2022, iyab155, https://doi.org/10.1093/genetics/iyab155
Close - Share Icon Share
Abstract
Zinc cluster transcription factors (TFs) are essential fungal regulators of gene expression. In the pathogen Candida albicans, the gene orf19.1604 encodes a zinc cluster TF regulating filament development. Hyperactivation of orf19.1604, which we have named RHA1 for Regulator of Hyphal Activity, generates wrinkled colony morphology under nonhyphal growth conditions, triggers filament formation, invasiveness, and enhanced biofilm formation and causes reduced virulence in the mouse model of systemic infection. The strain expressing activated Rha1 shows up-regulation of genes required for filamentation and cell-wall-adhesion-related proteins. Increased expression is also seen for the hyphal-inducing TFs Brg1 and Ume6, while the hyphal repressor Nrg1 is downregulated. Inactivation of RHA1 reduces filamentation under a variety of filament-inducing conditions. In contrast to the partial effect of either single mutant, the double rha1 ume6 mutant strain is highly defective in both serum- and Spider-medium-stimulated hyphal development. While the loss of Brg1 function blocks serum-stimulated hyphal development, this block can be significantly bypassed by Rha1 hyperactivity, and the combination of Rha1 hyperactivity and serum addition can generate significant polarization even in brg1 ume6 double mutants. Thus, in response to external signals, Rha1 functions with other morphogenesis regulators including Brg1 and Ume6, to mediate filamentation.
Introduction
Transcriptional control of cellular processes is critical for normal functioning of the medically important opportunistic fungal pathogen, Candida albicans. Central to this regulation is the DNA-binding transcription factors (TFs) that typically associate with target sequences in the promoters of regulated genes. The bound factors serve to activate or repress transcription in response to signals that generally represent either internal cellular states or external conditions (Lee and Young 2000; Kornitzer 2019). One of the main classes of C. albicans TFs are the zinc cluster proteins, named because of a cysteine-rich region that coordinates two zinc atoms as part of the DNA binding domain of the protein (MacPherson et al. 2006). Such zinc cluster transcription factors (ZCFs) are found only in fungi and amoebae, but related zinc finger proteins serve as TFs throughout eukaryotes (MacPherson et al. 2006; Clarke et al. 2013) Many studies have been conducted to characterize ZCF function; a powerful tool in these studies has been the use of activated versions of the ZCFs. For example, screening by Schillig and Morschhäuser (2013) of a comprehensive collection of Zn(II)2 Cys6 gain-of-function mutants identified regulatory genes controlling fluconazole resistance, including Mrr2 (multidrug resistance regulator) that regulates the multidrug efflux pump Cdr1. Tebung et al. (2016) used ZCF activation during the characterization of an example of rewiring between purine and pyrimidine metabolism in the ascomycetes, and recently in establishing a conserved role for Put3 in regulating proline catabolism in both C. albicans and Saccharomyces cerevisiae (Tebung et al. 2017). These studies led us to investigate ZCFs further to uncover the transcriptional regulatory circuits involved in pathogenicity-critical processes such as hyphal growth.
An essential characteristic of C. albicans critical for its success as a commensal and opportunistic pathogen is its ability to grow in different morphological forms. The two most common cellular forms are the yeast form, where cells grow by budding as individual, rounded cells, and the hyphal form, where cells grow as extended, branching filaments, with individual cells delineated by septa (Whiteway and Bachewich 2007). C. albicans cells use the filament form to escape from human macrophages and to invade into deeper tissues during infection (Rooney and Klein 2002; Kumamoto and Vinces 2005; Mayer et al. 2013). Transcriptional control is essential for the ability of cells to make the switch between the yeast and filamentous forms. Several TFs have been identified with functions in this switch. These include Efg1, a target of the Ras1/cAMP pathway (Stoldt et al. 1997), and Cph1, a target of the MAP kinase pathway (Liu et al. 1994), both being essential elements of the specific signal transduction pathways. They also include transcriptional repressors such as Tup1 (Braun and Johnson 1997) and Nrg1 (Braun et al. 2001).
The molecular mechanism of hyphal initiation suggests that environmental stimuli such as serum, Spider medium, and GlcNAc, serve to trigger a switch from a yeast state, where the Nrg1 repressor blocks activity of hyphal-associated genes (HAGs), to a hyphal state, where the Brg1 TFs and the associating Hda1 histone deacetylase remodel the chromatin state of the HAG promoters. This remodeling leads to the activation of the hyphal program (Lu et al. 2011). Thus, hyphal development involves downregulation of TF Nrg1 by the cAMP-dependent protein kinase A (PKA) pathway, together with upregulation of Brg1 and Ume6 (Braun et al. 2001), and it has been proposed that the chromatin state of the promoters of crucial genes represents a central regulator of transcriptional control of the transition between the yeast and hyphal states (Lu et al. 2012). This circuit regulating chromatin structure required for hyphal initiation and extension involves the GATA family member Brg1 (Lu et al. 2011) and Ume6, a zinc cluster family member (Banerjee et al. 2008; Zeidler et al. 2009). Brg1 expression is repressed by Nrg1 (Cleary et al. 2012), and Brg1 and Nrg1 compete to control the chromatin state.
Our work identifies a new TF involved in the hyphal transition. The zinc cluster TF Rha1 interacts with the Nrg1/Brg1 switch; activated Rha1 can trigger filamentous growth in the absence of external signals, and in the presence of external signals like serum, Rha1 activation can bypass the need for Brg1. Loss of Rha1 function leads to a reduced ability to generate hyphal growth in the presence of external signals, and when coupled with loss of Ume6 function, creates completely nonhyphal cells. This establishes Rha1 as a critical ZCF functioning in the hyphal control circuitry of the human pathogen C. albicans.
Materials and methods
Strains, media, and growth conditions
All C. albicans strains, oligonucleotides, used in this study are listed in Supplementary Tables S1 and S2. For long-term storage, cells were kept at −80°C in 25% glycerol supplemented YPD. The strains were routinely cultured in liquid YPD (10 g yeast extract, 20 g peptone, 20 g glucose per liter, 50 μg/ml uridine) (2% w/v Bacto-agar for solid medium) at 30°C in a shaking incubator. The C. albicans transformations were plated on YPD agar plates containing 200 µg/ml nourseothricin (Werner Bioagents, Jena, Germany) or 500–600 µg/ml Hygromycin B (HygB) (Bioshop Inc, Canada), for 48 h at 30°C.
Candida albicans mutant strains
All C. albicans mutants except ChIP-chip strain were constructed in the wild-type (WT) strain SC5314 (Gillum et al. 1984) using the lithium acetate method of transformation (Gietz et al. 1995) or electroporation (Köhler et al. 1997). Two Rha1 mutants were created using different approaches in strain SC5314. The rha1−/− strain was constructed by using the CRISPR Cas9 method to insert tandem stop codons into both RHA1 alleles. The rha1Δ/Δ mutant is the result of the deletion of both RHA1 alleles, giving rise to the rha1Δ/Δ mutant SC1604M4B. A CRISPR/Cas9 system (Vyas et al. 2015) was used to construct the RHA1 mutation strain. The sgRNA of RHA1 was formed by annealing primers RHA1-sg-F and RHA1-sg-R and cloning the fragment into the BsmBI site of pV1093 to make plasmid pV1093-Rha1-sgRNA. A repair DNA was generated using Rha1-Rep-F, and Rha1-Rep-R primers to PCR amplify a fragment containing in-frame stop codons with a disrupted PAM region and directing introduction of an XhoI site for the confirmation of transformants with the correct insertions. The repair DNA template and linearized plasmid pV1093-Rha1-sgRNA were transformed, then transformants with the correct mutations were verified by PCR. We used a transient CRISPR/Cas9 system (Min et al. 2016) to delete C. albicans BRG1 and UME6 in the Rha1-GOF background strains. A Cas9 gene was amplified using a pV1093 plasmid and P7 and P8 standard primers. The final sgRNA fragment was amplified using P5 and P6 primers from the product of two separate PCR reactions, including the sgRNA sequence. The Hygromycin B repair template was amplified from pYM70 plasmid (Basso et al. 2010) as a selection marker to create homozygous null mutants brg1Δ/Δ and ume6Δ/Δ. Correct deletions were confirmed using primers internal to the HygB markers in combination with primers for the upstream regions of UME6 or BRG1 and by using primers internal to the BRG1 and UME6 ORFs. The double mutant strains rha1−/− ume6Δ/Δ and rha1−/− brg1Δ/Δ were constructed in the rha1−/− NatR strain background. An rha1 deletion mutant of strain SC5314 was also generated by the SAT1-flipping method (Reuss et al. 2004). For this, RHA1 upstream and downstream sequences were amplified with the primer pairs orf19.1604-5‘FW/orf19.1604-5’RV and orf19.1604-3‘FW/orf19.1604-3’RV, respectively, and cloned on both sides of the SAT1 flipper cassette in plasmid pSFS5 (Sasse et al. 2011) The insert from the resulting plasmid p1604M1 was used to sequentially delete the two RHA1 alleles of strain SC5314, generating the rha1Δ/Δ mutant SC1604M4B. For reinsertion of an intact RHA1 copy, the RHA1 coding and flanking sequences were amplified with primers 1604-5‘FW-Kompl and 1604-3‘RV-Kompl and substituted for the RHA1 upstream flanking sequence in p1604M1 to yield p1604K2. The insert from this plasmid was used to reintegrate RHA1 into its endogenous locus in the rha1Δ/Δ mutant SC1604M4B, followed by recycling of the SAT1 flipper cassette to generate the complemented strain SC1604MK2B. A ume6Δ/Δ mutant of strain SC5314 was constructed in an analogous fashion. UME6 upstream and downstream sequences were amplified with the primer pairs UME6-ko1/UME6-ko2 and UME6-ko3/UME6-ko4, respectively, and cloned on both sides of the SAT1 flipper cassette in plasmid pSFS5. The insert from the resulting plasmid pUME6M2 was used to sequentially delete the two UME6 alleles of strain SC5314, generating the ume6Δ/Δ mutant SCUME6M4A. We constructed the RHA1-TAP strain from the strain SN148 (Noble and Johnson 2005; Lavoie et al. 2008) by transformation using a TAP-URA3 PCR product containing 99 bp of homologous sequence immediately upstream and downstream of the RHA1 stop codon. The TAP-URA3 portion of the oligomer used for transformation was amplified from the pFA-TAP-URA3 plasmid.
Filamentation assays
The environmental filament induction assays were performed by growing the selected strains overnight in liquid YPD at 30°C, 220-rpm. The next day, the cells were washed twice with 1× PBS and resuspended at OD600 = 0.1 into fresh liquid Spider (1.35% agar for solid medium, 1% nutrient broth, 0.4% potassium phosphate, and 2% mannitol, pH 7.2), YPD, or YPD plus 20% serum (Fetal Bovine serum; Sigma) then incubated at 37°C for 4 h. For solid medium, 5 µl from an OD600 = 0.1 culture was spotted onto YPD plus 20% serum plates and incubated at 37°C for 3 days.
Biofilm assays
Strains were grown overnight in 5 ml liquid YPD at 30°C on a shaker at 220 rpm. In the morning, after washing the cells twice with 1× PBS, the cell densities were adjusted to an OD600 = 0.5 in 200 μl Spider medium and then were added to a 96 well plate at 37°C for 1.5 h in static condition to allow cell adherence to the surface of the wells. Next, nonadherent cells were removed by washing with PBS and 200 μl of fresh Spider was added to each of the washed wells. The plate was incubated for 24 h at 37°C in a shaker at 75 rpm. Each well was washed twice with 200 μl of PBS, and the level of biofilm formation was determined by using the crystal violet assay, as reported previously (Daniels et al. 2013).
Stress assays
For stress assays, single colonies from WT and Rha1-GOF strains were grown overnight in YPD at 30°C and resuspended in 1× PBS to a cell density of 1.5 × 10 7 cells/ml at 600 nm, then were diluted in 10-fold stages from 106 to 102 in 1× PBS. These 10-fold serial dilutions of each strain were spotted onto YPD plates containing 5 mM hydrogen peroxide, 0.02% MMS, 10 mM FeSO4, 20 mM FeCl3, and 5 mM CuSO4, 0.4 M CaCl2, 1 M sorbitol, 0.15 mM menadione, 38 mM hydroxyurea, pH 10, 250 mM Glycerol, or 100 µg/ml Hygromycin B. Plates were incubated for 3 days at 30°C. The chemical concentrations were selected based on recent literature values used in large scale phenotypic screenings.
Microscopy and imaging
Single colonies of C. albicans strains were inoculated in 5 mL of liquid YPD and incubated overnight at 30°C with shaking at 220 rpm. The cells were then washed twice with 1× PBS and imaged using differential interference contrast (DIC). For the hyphal induction images, cells were washed twice with 1× PBS, followed by the addition of Calcofluor white stain (2 μg/ml; Sigma, USA) for 20 min and imaged as described below. For quantification of elongation factor, cells were stained with Calcofluor white stain, then imaged using a Leica DM6000 microscope with both DIC optics and a DAPI filter cube (377/50ex, 447/60em), using a 100x (N.A. 1.3), 60x (N.A. 1.4) or 40x (N.A. 0.75) objective lens and a Hamamatsu Orca R2 camera. After capture, Calcofluor white stain images were presented to a Region-based Convolutional Neural Net (R-CNN, He et al. 2017) trained to recognize yeast cells, resulting in a binary mask that represents the outline of most cells in the image; these masks were verified by a trained human observer, who could discard inappropriate masks that did not correlate well with merged DIC and Calcofluor white stain images. The remaining masks were measured in FIJI (NIH, Bethesda), using the Shape Descriptors option to extract the aspect ratio of each cell, being the ratio of the width of the cell to its height (Lu et al. 2019). The aspect ratio of filament cells was calculated for each compartment of the filament. Of note, this R-CNN was not trained on elongated cells and failed to find the most elongated hyphal cells, resulting in an underestimation of aspect ratio in samples containing extremely elongated cells. As outlined in the text, Kruskal-Wallis and Dunn's multiple comparisons tests were conducted on the data to determine statistical significance. GraphPad Prism 6.00 was used for analysis.
Invasion assays
Single colonies of the selected strains were grown overnight in 5 mL YPD at 30°C, 220 rpm. Then the cells were washed twice with 1× PBS and diluted to an optical density of OD600 = 0.1. Five micro litter from the adjusted cell density were spotted on YPD, incubated for 1 day at 30°C or when spotted on Spider agar, incubated at 37°C, 5 days. The spots on the Spider plates were then washed with sterile water for 15 s. Plates were scanned before and after washing using an Epson Perfection V500 Photo color scanner.
RNA isolation and RNA-seq experiment
Two biological replicates of both Rha1-GOF and WT strains were diluted to OD600 = 0.1 in YPD and inoculated to reach OD600 = 0.8–1. Total RNA was extracted using QIAGEN RNA extraction kit, then RNA quality and quantity were determined using an Agilent bioanalyzer. Paired end 150 bp illumine misSEQ sequencing was carried out at the Quebec Genome Innovation center. Raw reads were pre-processed as described previously (Costa et al. 2019). Briefly, the sequence-grooming tool cutadapt version 0.4.1 (Martin 2011) with the following quality trimming and filtering parameters (`–phred33 –length 36 -q 5 –stringency 1 -e 0.1`) was used. Each set of paired-end reads was mapped against the C. albicans SC5314 haplotype A version A22 downloaded from the Candida Genome Database (CGD) (http://www.candidagenome.org/) using HISAT2 version 2.0.4 (Kim et al. 2015). SAMtools (Li et al. 2009) was then used to sort and convert SAM files. The read alignments and SC5314 genome annotation were provided as input into StringTie v1.3.3 (Pertea et al. 2015), which returned gene abundances for each sample. Raw and processed data have been deposited in NCBI's Gene Expression Omnibus under accession number GSE143825 (Edgar et al. 2002). We imported gene abundances into R using the tximport R package and conducted differential analysis of transcript count data using the DESeq2 R package (Soneson et al. 2015). We use the independent hypothesis weighting (IHW) Bioconductor package (Ignatiadis et al. 2016) to weight P-values and adjust for multiple testing using the procedure of Benjamini Hochberg (BH) (Benjamini and Hochberg 1995). Expression heatmaps of selected genes were plotted using the pheatmap R package (https://cran.r-project.org/web/packages/pheatmap/index.html). Hierarchical clustering based on Euclidean distance and average linkage was applied to both samples (in columns) and genes (in rows). Gene ontology (GO) analyses were achieved on gene lists obtained by RNA-seq to identify putative enriched biological functions, processes, or cell compartments (Candida Genome Database GO Term Finder; http://www.candidagenome.org/cgi-bin/GO/goTermFinder) and to assign differentially regulated genes to specific biological processes (Candida Genome Database GO Slim Mapper; http://www.candidagenome.org/cgi-bin/GO/goTermMapper).
ChIP-chip
ChIP-chip experiments were performed as described previously (Tebung et al. 2016) with minor changes. Briefly, the strain containing the chromosomally integrated orf19.1604-TAP fusion, as well as the background strain SN148 (untagged), were grown to an OD600 of 0.6 in 50 ml of YPD medium and then used for ChIP. Cross-linking for each 50 ml culture was carried out in 1.5 ml of 37% formaldehyde for 30 min, and then ChIP was performed as described previously (Tebung et al. 2016). ChIP DNA extracted from tagged strains was labeled with Cy5 dye, ChIP DNA from untagged strain SN148 was labeled with Cy3 dye, and the samples were then cohybridized to Agilent 8X15K whole-genome arrays containing 14490 60-mer intergenic and intragenic oligonucleotide probes. Microarray hybridization, washing, scanning, and normalization were performed as described previously (Nantel et al. 2006) with the following modifications: The Axon GenePix 4000B microarray scanner was used to perform scanning, and GenePix data analysis software and Multiexperiment Viewer (MeV) software were used to analyze and normalize data; a 0.05 P-value cut-off was used for MeV analyses. The scanning settings used were 635 nm for Cy5 and 532 nm for Cy3. The log of ratios of Cy5 to Cy3 (635/532 nm) with a cut-off of at least 1.5 for each spot was considered to be an indicator of significant orf19.1604 binding.
Virulence studies
Male ICR mice of 6 weeks age (n = 12) were used for virulence assay as previous described (Feng et al. 2020). Briefly, 200 μl of 5 × 106 cells ml−1 were injected intravenously into the tail vein of mice. Survival rates were checked daily and survival curves were generated according to the Kaplan–Meier method using the PRISM program (version 5.0; GraphPad Software) and compared using the log-rank test. Moribund mice were sacrificed and sections were prepared from the kidneys and stained with periodic acid-Schiff’s (PAS) stain for histological examination (Feng et al. 2020). Mouse studies were carried out under the guidelines established by the Ethics Committee of Nantong University, China.
Results
Activation of Rha1 triggers Candida albicans filamentation
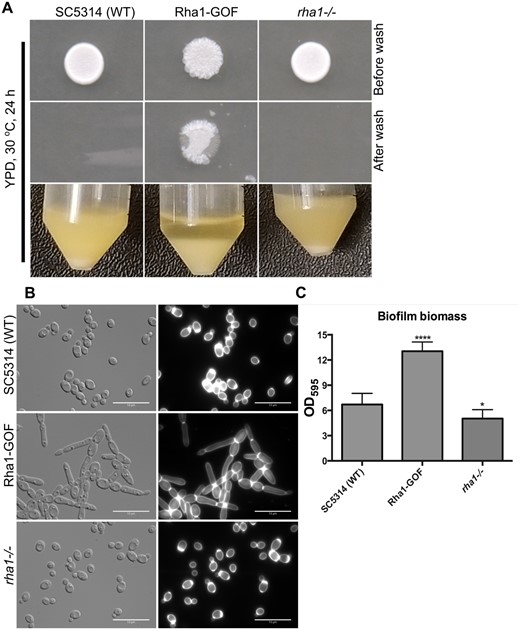
We screened a library of C. albicans strains containing overexpressed and activated zinc cluster TFs (Schillig and Morschhäuser 2013) and identified orf19.1604 as generating an abnormal colony morphology; colonies of cells over-expressing orf19.1604 fused to a Gal4 activation domain were wrinkled and crenulated whereas the WT colonies were smooth. The strains expressing the activated orf19.1604 were also invasive and resistant to washing from the surface of YPD medium plates after growth at 30°C. By contrast, cells of the WT were washed easily from the surface of the agar (Figure 1A). When grown in liquid YPD medium at 30°C, strains containing activated orf19.1604 were highly flocculent (Figure 1A), and assessment of the cellular morphology showed a high frequency of filamentous cells exhibiting primarily pseudohyphal characteristics (Figure 1B). As a consequence of this role in filamentation, we named the gene encoding orf19.1604 as RHA1, for Regulator of Hyphal Activity. Furthermore, we observed enhanced biofilm formation in the RHA1 activated strain compared to the WT strain (Figure 1C).
Morphological and biofilm results of the WT, Rha1-GOF, and rha1 mutant. (A) WT, Rha1-GOF, and rha1−/− strains were spotted on the solid nonfilament-inducing medium YPD and grown for 24 h before washing with a stream of water for 15 s. The Rha1-GOF strain was invasive and resistant to washing. The indicated strains were grown in liquid YPD medium at 30°C. Rha1-GOF cells are flocculent in YPD medium (B) The cellular morphology of the indicated strains is displayed after growth overnight at 30°C in liquid YPD medium. Cells were washed twice with 1× PBS, stained with CFW, and visualized by DIC optics (Bar, 10 μm). (C) A biofilm assay was run in biological triplicate in Spider medium at 37°C after 48 h. *P < 0.05, ***P < 0.001 relative to the WT strain (one-way ANOVA with Dunnet test multiple comparison test).
Rha1 orthologs are limited to the CTG clade
In C. albicans, RHA1 (C2_09460C_A) encodes a 989 amino acid protein with a zinc cluster type DNA-binding domain at the N-terminus that shows limited sequence similarity to that of Lys14 in S. cerevisiae, although Rha1 is unlike ScLys14 in the rest of the protein. In order to identify orthologs of C. albicans Rha1, the protein sequence from the Candida Genome Database (CGD) (http://www.candidagenome.org/) was compared across the ascomycetes using Blastp searches of the multiple fungal genome database (https://www.yeastgenome.org/blast-fungal). Convincing orthologs with sequence similarity outside of the DNA binding region of Rha1 are limited to CTG clade-specific species. They are not present in several phylogenetically related nonpathogenic species, including S. cerevisiae, S. paradoxus, S. mikatae, S. bayanus, S. castelli, and K. lactis.
Activated Rha1 does not influence general stress phenotypes
We examined the sensitivity of strains expressing activated Rha1 to a variety of stresses. Hyperactivity of Rha1 (Rha1-GOF) did not enhance sensitivity to the DNA damaging agent MMS at 0.02%, the oxidative stress-inducing agent hydrogen peroxide (H2O2) at 5 mM, the metal stressors 10 mM FeSO4, 20 mM FeCl3, and 5 mM CuSO4, and the osmotic stressor 1 M sorbitol. As well, cells were not sensitive to 0.4 M CaCl2, 0.15 mM menadione, 38 mM hydroxyurea, pH 10, glycerol at 250 mM, and Hygromycin B at 100 µg/ml (Supplementary Figure S1).
Activated Rha1 modulates gene expression
We performed RNA sequencing to determine the transcriptional profile of cells carrying the activated RHA1 allele (n = 2) compared to the WT cultured under the nonhyphae-inducing conditions of YPD medium at 30°C (n = 2). Three notable classes of genes were up-regulated above fourfold with adjusted P-value < 0.001, compared to the reference strain SC5314 (Table 1; Supplementary Figure S2A). The first class of genes encodes classic cell wall adhesins such as Als1, Als3, and hypha-related proteins including Hgc1, Hwp1, Ece1, and Hgt1, consistent with the observed phenotype of filamentous cells (Martin et al. 2013). A second class consists of genes encoding TFs that themselves regulate hyphal development, specifically Brg1 and Ume6. The third notable collection of significantly upregulated genes encodes arginine metabolism enzymes such as Arg1, Arg3, Arg4, and the arginine permease Can2. Among the top 35 downregulated genes, there is the well-studied hyphal repressor Nrg1, which is repressed 2.5-fold in Rha1-GOF cells. Interestingly, a number of TFs of the ZCF class including ZCF1, ZCF3, and FCR1, appear among the downregulated genes (Supplementary Figure S2B). Overall, the downregulated genes in Rha1-GOF compared to WT were enriched for biological function in transcription regulator activity. As well, Rha1-GOF down-regulated the arginine catabolism gene, CAR1, consistent with the high proportion of arginine biosynthesis genes upregulated in Rha1-GOF cells. A GO analysis indicated that the upregulated genes in Rha1-GOF were involved significantly in carbohydrate transport (35.8%), biological process involved in interspecies interaction between organisms (20%), regulation of growth (16.8%), biological adhesion (14.7%), filamentous growth (29.4%) and biofilm formation (27.4%) (Supplementary Table S3). These groupings were not mutually exclusive—many genes fell into more than one class.
RNAseq result of Rha1-GOF
| Gene . | Name . | log2FC . | Adjusted P-value . | Description . |
|---|---|---|---|---|
| orf19.3981 | MAL31 | 10 | 3.29E-09 | Putative high-affinity maltose transporter |
| orf19.4936.1 | - | 9.13 | 6.32E-07 | Putative adhesin-like protein; Spider biofilm induced |
| orf19.4527 | HGT1 | 9.03 | 5.85E-07 | High-affinity MFS glucose transporter |
| orf19.3374 | ECE1 | 9.02 | 1.18E-11 | Candidalysin; hypha-specific protein |
| orf19.113 | CIP1 | 8.55 | 1.57E-04 | Possible oxidoreductase |
| orf19.6028 | HGC1 | 7.8 | 2.02E-04 | Hypha-specific G1 cyclin-related protein |
| orf19.1822 | UME6 | 7.73 | 3.32E-04 | Zn(II)2Cys6 TF; role in hyphal extension |
| orf19.1227 | ZCF4 | 7.63 | 3.10E-04 | Putative Zn(II)2Cys6 TF |
| orf19.7469 | ARG1 | 7.21 | 1.07E-152 | Argininosuccinate synthase; arginine synthesis |
| orf19.1321 | HWP1 | 7.03 | 5.92E-11 | Hyphal cell wall protein |
| orf19.5610 | ARG3 | 5.56 | 9.49E-69 | Putative ornithine carbamoyltransferase |
| orf19.5741 | ALS1 | 5.38 | 2.23E-40 | Cell-surface adhesin; adhesion |
| orf19.4630 | CPA1 | 5.26 | 1.12E-82 | Putative carbamoyl-phosphate synthase subunit |
| orf19.6689 | ARG4 | 5.11 | 8.33E-84 | Argininosuccinate lyase |
| orf19.4377 | KRE1 | 5 | 6.96E-24 | Cell wall glycoprotein; beta glucan synthesis |
| orf19.7610 | PTP3 | 4.95 | 5.97E-82 | Putative protein tyrosine phosphatase; hypha induced |
| orf19.4056 | BRG1 | 4.82 | 8.10E-33 | TF; recruits Hda1 to hypha-specific promoters |
| orf19.3770 | ARG8 | 4.8 | 1.09E-60 | Putative acetylornithine aminotransferase |
| orf19.3221 | CPA2 | 4.74 | 7.76E-97 | Putative arginine-specific carbamoylphosphate synthetase |
| orf19.4788 | ARG5,6 | 4.62 | 3.21E-91 | Arginine biosynthetic enzyme |
| orf19.3282 | BMT3 | 4.48 | 5.13E-13 | Beta-mannosyltransferase |
| orf19.1816 | ALS3 | 4.42 | 2.62E-59 | Cell wall adhesin |
| Gene . | Name . | log2FC . | Adjusted P-value . | Description . |
|---|---|---|---|---|
| orf19.3981 | MAL31 | 10 | 3.29E-09 | Putative high-affinity maltose transporter |
| orf19.4936.1 | - | 9.13 | 6.32E-07 | Putative adhesin-like protein; Spider biofilm induced |
| orf19.4527 | HGT1 | 9.03 | 5.85E-07 | High-affinity MFS glucose transporter |
| orf19.3374 | ECE1 | 9.02 | 1.18E-11 | Candidalysin; hypha-specific protein |
| orf19.113 | CIP1 | 8.55 | 1.57E-04 | Possible oxidoreductase |
| orf19.6028 | HGC1 | 7.8 | 2.02E-04 | Hypha-specific G1 cyclin-related protein |
| orf19.1822 | UME6 | 7.73 | 3.32E-04 | Zn(II)2Cys6 TF; role in hyphal extension |
| orf19.1227 | ZCF4 | 7.63 | 3.10E-04 | Putative Zn(II)2Cys6 TF |
| orf19.7469 | ARG1 | 7.21 | 1.07E-152 | Argininosuccinate synthase; arginine synthesis |
| orf19.1321 | HWP1 | 7.03 | 5.92E-11 | Hyphal cell wall protein |
| orf19.5610 | ARG3 | 5.56 | 9.49E-69 | Putative ornithine carbamoyltransferase |
| orf19.5741 | ALS1 | 5.38 | 2.23E-40 | Cell-surface adhesin; adhesion |
| orf19.4630 | CPA1 | 5.26 | 1.12E-82 | Putative carbamoyl-phosphate synthase subunit |
| orf19.6689 | ARG4 | 5.11 | 8.33E-84 | Argininosuccinate lyase |
| orf19.4377 | KRE1 | 5 | 6.96E-24 | Cell wall glycoprotein; beta glucan synthesis |
| orf19.7610 | PTP3 | 4.95 | 5.97E-82 | Putative protein tyrosine phosphatase; hypha induced |
| orf19.4056 | BRG1 | 4.82 | 8.10E-33 | TF; recruits Hda1 to hypha-specific promoters |
| orf19.3770 | ARG8 | 4.8 | 1.09E-60 | Putative acetylornithine aminotransferase |
| orf19.3221 | CPA2 | 4.74 | 7.76E-97 | Putative arginine-specific carbamoylphosphate synthetase |
| orf19.4788 | ARG5,6 | 4.62 | 3.21E-91 | Arginine biosynthetic enzyme |
| orf19.3282 | BMT3 | 4.48 | 5.13E-13 | Beta-mannosyltransferase |
| orf19.1816 | ALS3 | 4.42 | 2.62E-59 | Cell wall adhesin |
Duplicated cultures of WT and Rha1-GOF strains were grown overnight and processed for RNA seq. Upregulated classes of genes identified include genes involved in Candida albicans filament formation, hyphal-specific TFs, and arginine biosynthesis. To be classified as up-regulated or down-regulated, RNAs must show a fourfold change in abundance with P-values <0.001.
RNAseq result of Rha1-GOF
| Gene . | Name . | log2FC . | Adjusted P-value . | Description . |
|---|---|---|---|---|
| orf19.3981 | MAL31 | 10 | 3.29E-09 | Putative high-affinity maltose transporter |
| orf19.4936.1 | - | 9.13 | 6.32E-07 | Putative adhesin-like protein; Spider biofilm induced |
| orf19.4527 | HGT1 | 9.03 | 5.85E-07 | High-affinity MFS glucose transporter |
| orf19.3374 | ECE1 | 9.02 | 1.18E-11 | Candidalysin; hypha-specific protein |
| orf19.113 | CIP1 | 8.55 | 1.57E-04 | Possible oxidoreductase |
| orf19.6028 | HGC1 | 7.8 | 2.02E-04 | Hypha-specific G1 cyclin-related protein |
| orf19.1822 | UME6 | 7.73 | 3.32E-04 | Zn(II)2Cys6 TF; role in hyphal extension |
| orf19.1227 | ZCF4 | 7.63 | 3.10E-04 | Putative Zn(II)2Cys6 TF |
| orf19.7469 | ARG1 | 7.21 | 1.07E-152 | Argininosuccinate synthase; arginine synthesis |
| orf19.1321 | HWP1 | 7.03 | 5.92E-11 | Hyphal cell wall protein |
| orf19.5610 | ARG3 | 5.56 | 9.49E-69 | Putative ornithine carbamoyltransferase |
| orf19.5741 | ALS1 | 5.38 | 2.23E-40 | Cell-surface adhesin; adhesion |
| orf19.4630 | CPA1 | 5.26 | 1.12E-82 | Putative carbamoyl-phosphate synthase subunit |
| orf19.6689 | ARG4 | 5.11 | 8.33E-84 | Argininosuccinate lyase |
| orf19.4377 | KRE1 | 5 | 6.96E-24 | Cell wall glycoprotein; beta glucan synthesis |
| orf19.7610 | PTP3 | 4.95 | 5.97E-82 | Putative protein tyrosine phosphatase; hypha induced |
| orf19.4056 | BRG1 | 4.82 | 8.10E-33 | TF; recruits Hda1 to hypha-specific promoters |
| orf19.3770 | ARG8 | 4.8 | 1.09E-60 | Putative acetylornithine aminotransferase |
| orf19.3221 | CPA2 | 4.74 | 7.76E-97 | Putative arginine-specific carbamoylphosphate synthetase |
| orf19.4788 | ARG5,6 | 4.62 | 3.21E-91 | Arginine biosynthetic enzyme |
| orf19.3282 | BMT3 | 4.48 | 5.13E-13 | Beta-mannosyltransferase |
| orf19.1816 | ALS3 | 4.42 | 2.62E-59 | Cell wall adhesin |
| Gene . | Name . | log2FC . | Adjusted P-value . | Description . |
|---|---|---|---|---|
| orf19.3981 | MAL31 | 10 | 3.29E-09 | Putative high-affinity maltose transporter |
| orf19.4936.1 | - | 9.13 | 6.32E-07 | Putative adhesin-like protein; Spider biofilm induced |
| orf19.4527 | HGT1 | 9.03 | 5.85E-07 | High-affinity MFS glucose transporter |
| orf19.3374 | ECE1 | 9.02 | 1.18E-11 | Candidalysin; hypha-specific protein |
| orf19.113 | CIP1 | 8.55 | 1.57E-04 | Possible oxidoreductase |
| orf19.6028 | HGC1 | 7.8 | 2.02E-04 | Hypha-specific G1 cyclin-related protein |
| orf19.1822 | UME6 | 7.73 | 3.32E-04 | Zn(II)2Cys6 TF; role in hyphal extension |
| orf19.1227 | ZCF4 | 7.63 | 3.10E-04 | Putative Zn(II)2Cys6 TF |
| orf19.7469 | ARG1 | 7.21 | 1.07E-152 | Argininosuccinate synthase; arginine synthesis |
| orf19.1321 | HWP1 | 7.03 | 5.92E-11 | Hyphal cell wall protein |
| orf19.5610 | ARG3 | 5.56 | 9.49E-69 | Putative ornithine carbamoyltransferase |
| orf19.5741 | ALS1 | 5.38 | 2.23E-40 | Cell-surface adhesin; adhesion |
| orf19.4630 | CPA1 | 5.26 | 1.12E-82 | Putative carbamoyl-phosphate synthase subunit |
| orf19.6689 | ARG4 | 5.11 | 8.33E-84 | Argininosuccinate lyase |
| orf19.4377 | KRE1 | 5 | 6.96E-24 | Cell wall glycoprotein; beta glucan synthesis |
| orf19.7610 | PTP3 | 4.95 | 5.97E-82 | Putative protein tyrosine phosphatase; hypha induced |
| orf19.4056 | BRG1 | 4.82 | 8.10E-33 | TF; recruits Hda1 to hypha-specific promoters |
| orf19.3770 | ARG8 | 4.8 | 1.09E-60 | Putative acetylornithine aminotransferase |
| orf19.3221 | CPA2 | 4.74 | 7.76E-97 | Putative arginine-specific carbamoylphosphate synthetase |
| orf19.4788 | ARG5,6 | 4.62 | 3.21E-91 | Arginine biosynthetic enzyme |
| orf19.3282 | BMT3 | 4.48 | 5.13E-13 | Beta-mannosyltransferase |
| orf19.1816 | ALS3 | 4.42 | 2.62E-59 | Cell wall adhesin |
Duplicated cultures of WT and Rha1-GOF strains were grown overnight and processed for RNA seq. Upregulated classes of genes identified include genes involved in Candida albicans filament formation, hyphal-specific TFs, and arginine biosynthesis. To be classified as up-regulated or down-regulated, RNAs must show a fourfold change in abundance with P-values <0.001.
To further investigate the targets of Rha1, we investigated the binding site of Rha1::TAP using ChIP-chip. This analysis identified a set of candidate target filament or biofilm-related genes like CEK1, BRG1, DEF1 (EED1) as well as other genes of unknown function (Table 2; Supplementary Table S4). Overall, the overlap in these candidate targets with transcriptionally up-regulated genes in the Rha1-GOF strain shows strong enrichment in genes induced during biofilm formation (Table 2).
Overlap summary between ChIP-chip and RNA-seq data
| Feature . | Orf . | Gene . | Description . |
|---|---|---|---|
| C1_08170C_A | orf19.5094 | BUL1 | Protein similar but not orthologous to S. cerevisiae Bul1 |
| C1_08940C_A | orf19.4752 | MSN4 | Zinc finger TF; similar to S. cerevisiae Msn4, but not a significant stress response regulator in C. albicans |
| C2_08920W_A | orf19.215 | — | Component of a complex containing the Tor2p kinase; possible a role in regulation of cell growth; Spider biofilm induced |
| C3_01800C_A | orf19.1666 | — | Ortholog of Dig2, a MAP kinase-responsive inhibitor of Ste12 |
| C4_02990C_A | orf19.2693 | GST2 | Glutathione S transferase; induced by benomyl and in populations of cells exposed to fluconazole over multiple generations |
| C4_06480C_A | orf19.2886 | CEK1 | ERK-family protein kinase; required for wild-type yeast-hypha switch, mating efficiency, virulence in mice |
| C5_03930C_A | orf19.3219 | — | Ortholog of S. cerevisiae Sia1 |
| C7_01390W_A | orf19.6920 | — | Protein of unknown function; induced during chlamydospore formation in both C. albicans and C. dubliniensis |
| C7_01680C_A | orf19.6556 | — | Protein of unknown function; rat catheter, flow model and Spider biofilm induced |
| CR_00290W_A | orf19.7504 | — | Ortholog of S. cerevisiae Rts3; a component of the protein phosphatase type 2A complex |
| CR_03890W_A | orf19.467 | WOR3 | TF; modulator of white-opaque switch; induced in opaque cells; promoter bound by Wor1 |
| CR_06790C_A | orf19.1855 | — | Predicted membrane transporter, member of the anion: cation symporter (ACS) family, major facilitator superfamily (MFS); Gcn4p-regulated |
| CR_09880W_A | orf19.7561 | DEF1 | RNA polymerase II regulator; role in filamentation, epithelial cell escape, dissemination in RHE model |
| C1_05140W_A | orf19.4056 | BRG1 | TF; recruits Hda1 to hypha-specific promoters; Tn mutation affects filamentation; Hap43-repressed |
| Feature . | Orf . | Gene . | Description . |
|---|---|---|---|
| C1_08170C_A | orf19.5094 | BUL1 | Protein similar but not orthologous to S. cerevisiae Bul1 |
| C1_08940C_A | orf19.4752 | MSN4 | Zinc finger TF; similar to S. cerevisiae Msn4, but not a significant stress response regulator in C. albicans |
| C2_08920W_A | orf19.215 | — | Component of a complex containing the Tor2p kinase; possible a role in regulation of cell growth; Spider biofilm induced |
| C3_01800C_A | orf19.1666 | — | Ortholog of Dig2, a MAP kinase-responsive inhibitor of Ste12 |
| C4_02990C_A | orf19.2693 | GST2 | Glutathione S transferase; induced by benomyl and in populations of cells exposed to fluconazole over multiple generations |
| C4_06480C_A | orf19.2886 | CEK1 | ERK-family protein kinase; required for wild-type yeast-hypha switch, mating efficiency, virulence in mice |
| C5_03930C_A | orf19.3219 | — | Ortholog of S. cerevisiae Sia1 |
| C7_01390W_A | orf19.6920 | — | Protein of unknown function; induced during chlamydospore formation in both C. albicans and C. dubliniensis |
| C7_01680C_A | orf19.6556 | — | Protein of unknown function; rat catheter, flow model and Spider biofilm induced |
| CR_00290W_A | orf19.7504 | — | Ortholog of S. cerevisiae Rts3; a component of the protein phosphatase type 2A complex |
| CR_03890W_A | orf19.467 | WOR3 | TF; modulator of white-opaque switch; induced in opaque cells; promoter bound by Wor1 |
| CR_06790C_A | orf19.1855 | — | Predicted membrane transporter, member of the anion: cation symporter (ACS) family, major facilitator superfamily (MFS); Gcn4p-regulated |
| CR_09880W_A | orf19.7561 | DEF1 | RNA polymerase II regulator; role in filamentation, epithelial cell escape, dissemination in RHE model |
| C1_05140W_A | orf19.4056 | BRG1 | TF; recruits Hda1 to hypha-specific promoters; Tn mutation affects filamentation; Hap43-repressed |
Five hundred and two of Rha1-GOF ChIP-chip > 1.5 log of ratio bound site and 106 Rha1-GOF RNAseq upregulated genes with log2FoldChange > 1.5 identified.
Overlap summary between ChIP-chip and RNA-seq data
| Feature . | Orf . | Gene . | Description . |
|---|---|---|---|
| C1_08170C_A | orf19.5094 | BUL1 | Protein similar but not orthologous to S. cerevisiae Bul1 |
| C1_08940C_A | orf19.4752 | MSN4 | Zinc finger TF; similar to S. cerevisiae Msn4, but not a significant stress response regulator in C. albicans |
| C2_08920W_A | orf19.215 | — | Component of a complex containing the Tor2p kinase; possible a role in regulation of cell growth; Spider biofilm induced |
| C3_01800C_A | orf19.1666 | — | Ortholog of Dig2, a MAP kinase-responsive inhibitor of Ste12 |
| C4_02990C_A | orf19.2693 | GST2 | Glutathione S transferase; induced by benomyl and in populations of cells exposed to fluconazole over multiple generations |
| C4_06480C_A | orf19.2886 | CEK1 | ERK-family protein kinase; required for wild-type yeast-hypha switch, mating efficiency, virulence in mice |
| C5_03930C_A | orf19.3219 | — | Ortholog of S. cerevisiae Sia1 |
| C7_01390W_A | orf19.6920 | — | Protein of unknown function; induced during chlamydospore formation in both C. albicans and C. dubliniensis |
| C7_01680C_A | orf19.6556 | — | Protein of unknown function; rat catheter, flow model and Spider biofilm induced |
| CR_00290W_A | orf19.7504 | — | Ortholog of S. cerevisiae Rts3; a component of the protein phosphatase type 2A complex |
| CR_03890W_A | orf19.467 | WOR3 | TF; modulator of white-opaque switch; induced in opaque cells; promoter bound by Wor1 |
| CR_06790C_A | orf19.1855 | — | Predicted membrane transporter, member of the anion: cation symporter (ACS) family, major facilitator superfamily (MFS); Gcn4p-regulated |
| CR_09880W_A | orf19.7561 | DEF1 | RNA polymerase II regulator; role in filamentation, epithelial cell escape, dissemination in RHE model |
| C1_05140W_A | orf19.4056 | BRG1 | TF; recruits Hda1 to hypha-specific promoters; Tn mutation affects filamentation; Hap43-repressed |
| Feature . | Orf . | Gene . | Description . |
|---|---|---|---|
| C1_08170C_A | orf19.5094 | BUL1 | Protein similar but not orthologous to S. cerevisiae Bul1 |
| C1_08940C_A | orf19.4752 | MSN4 | Zinc finger TF; similar to S. cerevisiae Msn4, but not a significant stress response regulator in C. albicans |
| C2_08920W_A | orf19.215 | — | Component of a complex containing the Tor2p kinase; possible a role in regulation of cell growth; Spider biofilm induced |
| C3_01800C_A | orf19.1666 | — | Ortholog of Dig2, a MAP kinase-responsive inhibitor of Ste12 |
| C4_02990C_A | orf19.2693 | GST2 | Glutathione S transferase; induced by benomyl and in populations of cells exposed to fluconazole over multiple generations |
| C4_06480C_A | orf19.2886 | CEK1 | ERK-family protein kinase; required for wild-type yeast-hypha switch, mating efficiency, virulence in mice |
| C5_03930C_A | orf19.3219 | — | Ortholog of S. cerevisiae Sia1 |
| C7_01390W_A | orf19.6920 | — | Protein of unknown function; induced during chlamydospore formation in both C. albicans and C. dubliniensis |
| C7_01680C_A | orf19.6556 | — | Protein of unknown function; rat catheter, flow model and Spider biofilm induced |
| CR_00290W_A | orf19.7504 | — | Ortholog of S. cerevisiae Rts3; a component of the protein phosphatase type 2A complex |
| CR_03890W_A | orf19.467 | WOR3 | TF; modulator of white-opaque switch; induced in opaque cells; promoter bound by Wor1 |
| CR_06790C_A | orf19.1855 | — | Predicted membrane transporter, member of the anion: cation symporter (ACS) family, major facilitator superfamily (MFS); Gcn4p-regulated |
| CR_09880W_A | orf19.7561 | DEF1 | RNA polymerase II regulator; role in filamentation, epithelial cell escape, dissemination in RHE model |
| C1_05140W_A | orf19.4056 | BRG1 | TF; recruits Hda1 to hypha-specific promoters; Tn mutation affects filamentation; Hap43-repressed |
Five hundred and two of Rha1-GOF ChIP-chip > 1.5 log of ratio bound site and 106 Rha1-GOF RNAseq upregulated genes with log2FoldChange > 1.5 identified.
Loss of Rha1 function modulates hyphal development
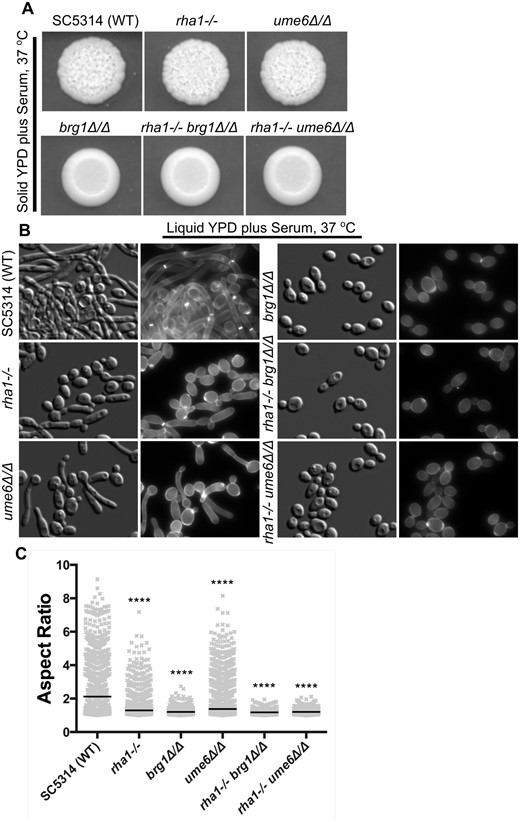
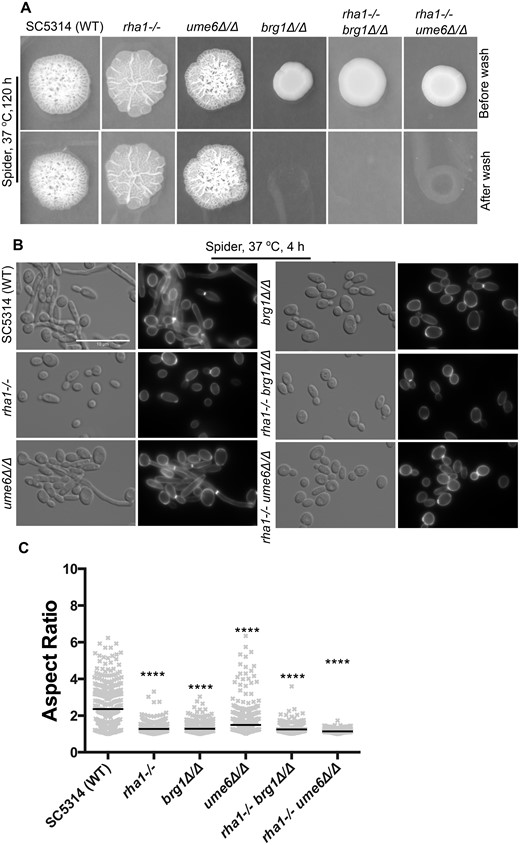
Activation of Rha1 leads to filamentation, and ChIP-chip and RNA-seq analysis showed a link to biofilm formation. We therefore asked if Rha1 function is required for normal hyphal development in response to hyphal inducing conditions. We inactivated both alleles of RHA1 in the WT background and assessed filamentation and biofilm formation. In comparison to the WT strain, the rha1−/− strain showed a 33% reduction in biofilm formation (Figure 1C). We assessed hyphal development in response to both serum medium and growth on Spider medium at 37°C. Colony morphology appeared unchanged on solid serum media (Figure 2A) and showed indistinguishable invasion on Spider medium (Figure 3A). However, the rha1−/− strain formed less inner colony wrinkling compared to the WT and ume6Δ/Δ (Figure 3A). Cell morphology was changed in response to growth in liquid serum and Spider medium. Under serum inducing conditions at 37°C at time 4 h, the mutants rha1−/−, brg1Δ/Δ and ume6Δ/Δ displayed a filamentation defect compared with the WT (Figure 2, B and C). As is shown in Figure 2C, the WT cells can filament as expected, with median aspect ratio (AR, a length to width ratio serving as a measure of cell polarity) of 2.1. Cells lacking Rha1 showed reduced filamentation (median AR = 1.3), as did mutant strains lacking the classic hyphal regulators Ume6 (slightly, median AR = 1.4) and Brg1 (dramatically, median AR = 1.2). The filamentation defect was more extreme for Spider induction conditions as shown in Figure 3B; here, the rha1 mutant strain was strongly compromised in hyphal development after 4 h at 37°C. The WT strain filamented with median AR of 2.4, but cells lacking Rha1 (AR median of 1.3), Brg1 (AR median of 1.3) and Ume6 (AR median of 1.5) showed significantly reduced filamentation compared to the WT (Figure 3C). Taken together, these results suggest that Rha1 plays a key role in filamentation in liquid serum and Spider medium.
Deletion of RHA1 and UME6 causes defects in filamentation under serum stimuli. (A) The wrinkled colony morphology of the rha1, ume6, and brg1 single mutants, and rha1−/− ume6Δ/Δ and rha1−/− brg1Δ/Δ double mutants, together with the WT strain SC5314 on 20% solid serum medium are shown after 3 days of growth at 37°C. (B) Strains were grown in liquid YPD supplemented with 20% serum for 4 h, 37°C, stained with CFW, and examined at 63x magnification by DIC optics and a DAPI filter cube (Bar, 10 µm). (C). Aspect ratio analysis of the cellular morphology of strains for each experimental group, N ≥ 1314 cells derived from at least 3 different experimental repeats. The horizontal lines indicate the median. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Kruskal–Wallis test with Dunn’s multiple comparison test.
Inactivation of RHA1 causes defects in hyphal formation on Spider medium. (A) The invasiveness of strains after growth on Spider medium for 120 h was tested after washing colonies with a stream of water. (B) The cellular morphology of the indicated strains grown in the liquid Spider medium, 4 h at 37°C. Scale bar is 10 µm. The rha1−/− ume6Δ/Δ double mutants were highly defective in hyphal formation when grown in Spider medium. (C) Aspect ratio analysis of Spider-treated strains is shown to quantify the reduction in filamentation. Horizontal lines show the median AR. n ≥ 315. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Kruskal–Wallis test with Dunn’s multiple comparison test.
We also assessed whether the rha1 inactivated strain was auxotrophic for arginine, because activation of Rha1 also leads to the up-regulation of genes involved in arginine metabolism (Table 1). We grew the strain in liquid SC-arg medium for 72 h at 30 °C. In the absence of arginine supplementation, the mutant strain grew identically to the control strain (Supplementary Figure S3). Finally, we tested complementation to further confirm Rha1 function by adding a single copy of RHA1 back to a rha1Δ/Δ mutant. The complemented strain rescued the rha1Δ/Δ mutant filamentation defects, confirming that the initial observed phenotype was due to loss of Rha1 function (Supplementary Figure S4).
Gain of function of Rha1 impairs virulence in a mouse model
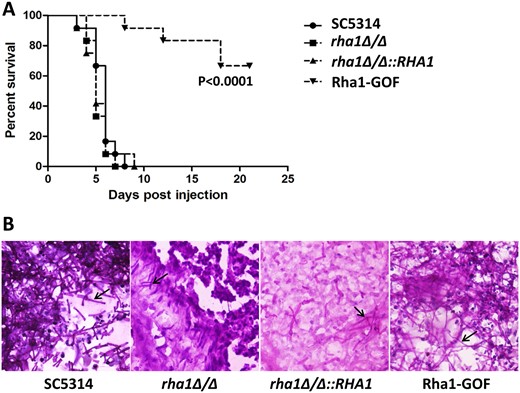
Given the role of Rha1 in hyphae-formation regulation, we checked the function of the TF in virulence by injecting the Rha1 gain-of-function strain and the rha1 deletion strain separately into mice. The mice infected with the WT cells all died by day 8, showing an average survival of 6 days, while the mice infected with the rha1 deletion cells and complemented strain both showed an average survival of 5 days, with no significant difference with the WT group (Figure 4A). However, the Rha1 gain-of-function strain showed an impaired virulence; only 4 mice infected with this strain died by day 18, a significant difference from the WT group. Consistently, in the kidneys of mice, WT cells, the rha1 deletion, the RHA1 complemented, and the Rha1 gain-of-function cells all showed filamentous forms (Figure 4B), although the null mutant infections appeared to have fewer of these forms. Overall, it appears that the gain-of-function allele of RHA1, but not the deletion, impairs virulence significantly compared to the WT.
Hyperactivity of Rha1 reduces virulence in mice. (A) Survival curves of mice intravenously infected with the indicated C. albicans strains. Male ICR mice (6 weeks old, 12 mice for each group) were injected with 1 × 106 stationary-phase cells. Mice were checked daily for morbidity. (B) Histopathological examination of kidney tissues of moribund mice was performed after sacrifice. The infected kidney tissues were stained with periodic acid-Schiff’s reagent. The hyphae cells were indicated by black arrows.
Brg1 and Ume6 are required for the Rha1 hyperactive phenotype
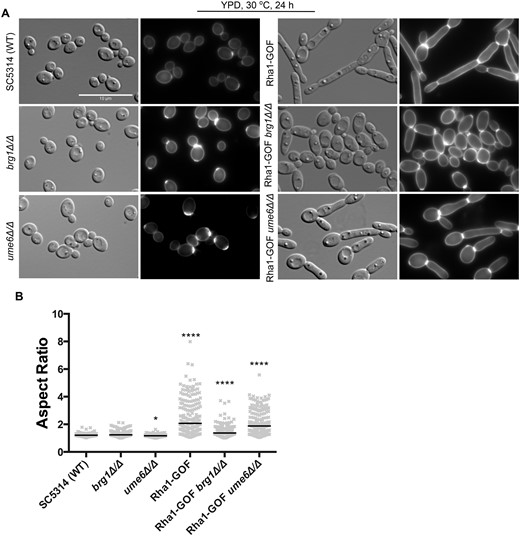
The expression profiling results showed that the key hyphal-regulator-encoding genes BRG1 and UME6 were overexpressed in strains with hyperactive Rha1. We investigated whether the function of either gene was required for the filamentous phenotype generated by Rha1 hyperactivation under normal yeast growth conditions (Figure 5A). The strain with the activated Rha1 construct shows an aspect ratio (median AR of 2.1). We deleted UME6 and BRG1 in this strain and observed a significant loss of induced filamentation in the Rha1-GOF brg1Δ/Δ strain, with the median of the aspect ratio dropping to 1.4, supporting a role of Brg1 in activated Rha1-triggered filamentation. There was much less reduction in filamentation in the Rha1-GOF ume6Δ/Δ strain (median AR= 1.9) (Figure 5B). As expected, the single mutants of Brg1 and Ume6 alone displayed normal yeast morphology with median ARs of 1.3 and 1.1, respectively. These results indicate that BRG1 contributes to proper filamentation in the Rha1 activated strain. In contrast, the UME6 deletion only reduces the hyphal extension in the Rha1 hyperactive strain, which is consistent with its defined role in the normal extension process (Banerjee et al. 2008).
Deletion of BRG1 and UME6 causes defects in Rha1-GOF-induced morphology. (A) Cellular morphology of the indicated strains under yeast growth conditions and imaged with DIC optics and DAPI filter cube (B) Horizontal lines represent the median AR. n ≥ 200. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Kruskal–Wallis test with Dunn’s multiple comparison test. Scale bar is 10 µm.
Rha1 and TFs controlling Candida albicans filamentation
We further investigated the relationship between Rha1 and the hyphal-controlled TFs identified in our profiling experiment. We first constructed double mutant combinations of the RHA1 with UME6 and BRG1 deletions to extend our examination of the effect of RHA1 on hyphal development in response to environmental stimuli. As shown in Figures 2B and 3B, the rha1 brg1 double mutant was not significantly different from the brg1 single mutant, which was by itself essentially nonhyphal in response to either serum or Spider inducing conditions (median ARs of 1.2 and 1.3, respectively). By contrast, however, the rha1 ume6 double mutant was significantly compromised in hyphal formation in response to either serum (median AR = 1.2) or Spider (median AR = 1.1) stimulation compared to the WT, even though the single mutants still showed significant morphological response to either hyphal inducing condition (Figures 2C and 3C). As previously noted, filamentation was reduced in ume6 single mutants under serum (median AR =1.4) and not significantly under Spider (median AR = 1.5) stimuli, and cells lacking Rha1 showed aspect ratio median of 1.3 under serum and Spider induced conditions.
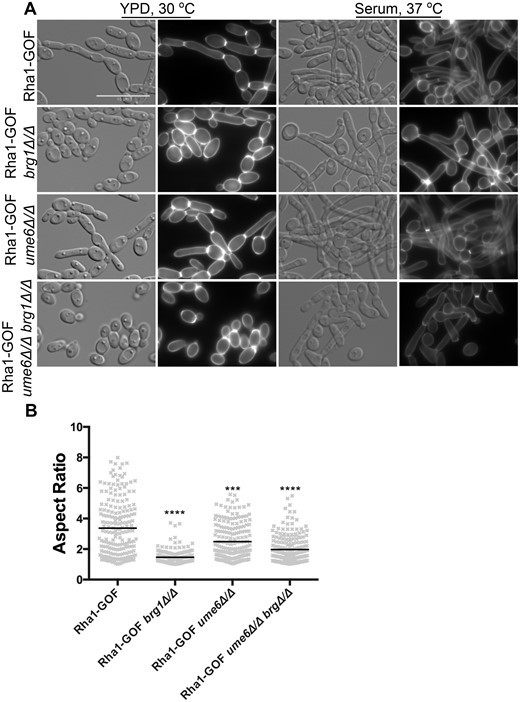
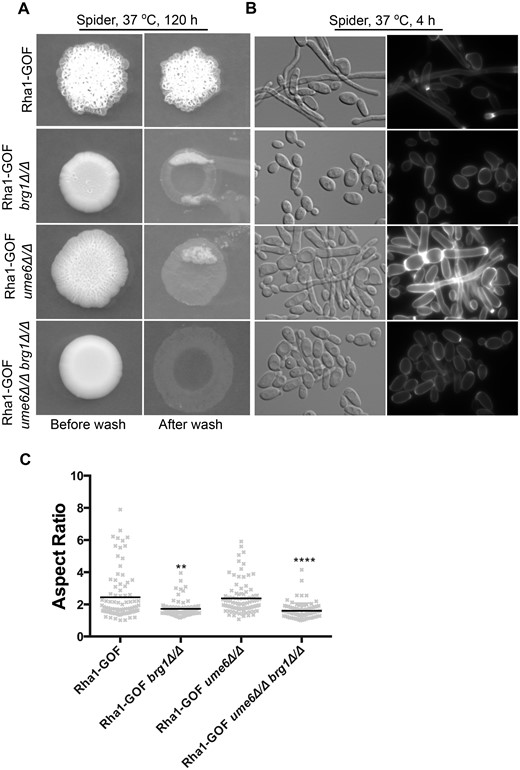
We also examined the effect of the Rha1 activated allele on the serum and Spider medium stimulation of hyphal development in the ume6 and brg1 null mutants. As can be seen in Figure 6, cells that were defective in hyphal formation in response to serum due to deletion of UME6 or BRG1 formed long filaments when the environmental stimulus was combined with the hyperactivated Rha1 construct. Serum treatment in the presence of the hyperactive Rha1 construct (median AR = 3.4) was able to significantly stimulate some cellular elongation even in cells deleted for both BRG1 and UME6 compared to the parental strain in yeast growth condition (Figure 6, A and B). Serum stimulation caused the Rha1-GOF ume6Δ/Δ strain to form filaments with median AR of 2.5, and even the Rha1-GOF brg1Δ/Δ strain to filament with a mean AR of 1.5 and Rha1-GOF ume6Δ/Δ brg1Δ/Δ strain (median AR = 2) (Figure 6B). As well, strains with activated Rha1 and BRG1 or UME6 deleted showed filaments on Spider medium but generated no wrinkled morphology on solid plates (Figure 7A and B). As is shown in Figure 7C, the Rha1-GOF brg1Δ/Δ strain incubated in Spider medium demonstrated cells lacking yeast morphologies (median AR = 1.7) compared to the Rha1-GOF (median AR = 2.4). However, Rha1-GOF ume6Δ/Δ formed elongated cells (median AR = 2.4), notably better than Rha1-GOF incubated in Spider medium (median AR = 2.4). Furthermore, Rha1-GOF cells lacking UME6 and BRG1 incubated in the same medium were not triggered to form filaments (median AR = 1.6) (Figure 7C). This suggests that while hyperactivation of Rha1 was capable of bypassing the requirement of Brg1 and Ume6 in serum-rich media to trigger filamentation, this response was less effective in Spider medium.
Rha1-GOF rescues filament development in brg1 and ume6 single mutants in the presence of 20% serum. (A) Overnight cultures of strains grown in YPD plus 20% serum (B) The aspect ratio of cells after 4 h in 20% serum and overexpression of Rha1. When supplemented with serum the Rha1-GOF could bypass the effect of brg1 and ume6 single mutants. The cells imaged by DIC and a DAPI filter cube. N ≥ 200; Horizontal lines represent the median AR. n ≥ 200. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Kruskal–Wallis test with Dunn’s multiple comparison test.
The media specificity of Rha1 for bypass of the filamentation defects of single brg1 and ume6 mutants. (A) Strains were spotted on Spider medium, and invasiveness assayed after being washed twice with 1× PBS and incubated 5 days. (B) DIC and fluorescence images represented cellular morphology of strains grown in liquid Spider medium. Rha1 overexpression coupled with Spider medium showed some morphological effects in the ume6Δ/Δ mutant but failed to bypass the phenotypes of brg1Δ/Δ or brg1Δ/Δ ume6Δ/Δ mutants. Horizontal lines represent the median AR. n ≥ 91. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Kruskal–Wallis test with Dunn’s multiple comparison test. Scale bar represents 10 µm.
Discussion
One of the critical characteristics that allows C. albicans to be a successful fungal pathogen is its capability of transitioning from a budding yeast to a filamentous form. C. albicans cells use this morphological plasticity to escape from human macrophages and to invade into deeper tissues during infection (Lo et al. 1997; Rooney and Klein 2002; Mayer et al. 2013). Numerous studies have identified a variety of signaling pathways, with a complex interconnected network of TFs, required for regulation of hyphae-associated genes (HAG) under different external stimuli (Biswas et al. 2007). These signaling pathways include the mitogen-activated protein kinase (MAPK), the target of rapamycin (TOR), the regulation of Ace2 morphogenesis (RAM), and the RAS/cAMP pathways (Basso et al. 2019).
The use of gene deletions to investigate the roles of individual members of such complex transcriptional regulatory circuits can be problematic in cases of functional redundancy (Kafri et al. 2006). As an alternative, artificial gene activation can be a powerful strategy to uncover specific gene functions and to relate a phenotype to genotype for functional discovery in such interlinked systems (Prelich 2012). In this study, we used a variety of approaches, including genetic activation, to explore the molecular role of a new member of the hyphal control circuit, a zinc cluster family member we have termed Rha1 for regulator of hyphal activity. The Rha1 protein is a classic zinc cluster TF; it is 989 amino acids long, with the zinc cluster DNA binding domain found from aa 16 to aa 47. Protein sequence alignments of CaRha1p revealed putative orthologs exclusively in the CTG clade of Saccharomycotina, whereas searches directed outside of the Candida clade show sequence similarity limited to the generally conserved zinc cluster DNA binding domain. The CTG group contains a large number of closely related pathogenic yeast, including C. dubliniensis, C. parapsilosis, C. tropicalis, C. lusitaniae, and C. guilliermondii, and filamentation has been connected with pathogenesis (Turner and Butler 2014). Activation of Rha1 established that it functions in the hyphal development circuit; transcriptional profiling and analysis of genetic interactions suggest that its role in this circuit connects it to chromatin structure through the Nrg1 repressor and the Brg1 and Ume6 activators.
Regulation of TFs such as Brg1 and Ume6 has been connected to a central signaling pathway controlling hyphal development that requires the cAMP-dependent PKA. PKA is activated by a cAMP signal created through Ras1 and adenylyl cyclase (Cdc35/Cyr1). The action of this pathway ultimately results in the relief of Nrg1-mediated repression of hyphal-associated genes (HAGs) (Lu et al. 2011, 2014). Overall, PKA regulates chromatin conformation by changing Nrg1-mediated repression into Brg1-mediated activation at the promoters of HAGs This transition also involves a subunit of the histone chaperone complex (Hir1), which has been shown to be involved in the downregulation of Nrg1 and initiation of hyphal development (Jenull et al. 2017), and the hyphal elongation TF Ume6, which functions during the hyphal maintenance phase (Lu et al. 2011).
Strains defective in Brg1 are unable to initiate hyphal development and remained locked in the yeast state in response to activating signals like serum (Homann et al. 2009; Cleary et al. 2012). Strains defective in Ume6 can initiate but not maintain induced hyphal development, and thus generate short germ tubes under inducing conditions (Banerjee 2008; Zeidler et al. 2009). However, loss of Brg1 or Ume6 were bypassed in serum-stimulated cells that contained a hyperactive allele of RHA1; hyphal development in response to serum proceeded efficiently, suggesting Rha1 hyperactivity can compensate for the loss of either Brg1 or Ume6. However, in the presence of hyperactivated Rha1, serum treatment of the brg1 ume6 double mutant triggered polarized growth but not extensive filaments, showing that Rha1 activation cannot altogether bypass the Brg1/Ume6 circuit. Intriguingly, on solid medium hyperactive Rha1 did not overcome the filamentation defect of the brg1 mutant in the presence of serum. Previous studies have established different genetic and transcriptional programs during hyphal development in cells responding to growth in either liquid or solid media (Azadmanesh et al. 2017).
In addition to bypassing blocks in serum-stimulated hyphal development in brg1 and ume6 mutants, we found that hyperactive Rha1 directs constitutive hyphal growth, creates an invasive phenotype in the absence of external hyphal inducing signals, and stimulates biofilm formation. Consistent with the phenotypic observations, transcriptional profiling of the hyperactivated Rha1 strain in the absence of external signals for hyphal development revealed induction of a variety of hyphal-specific genes like ECE1 and HWP1, as well as genes for the TFs Brg1 and Ume6, and also showed downregulation of the gene for the hyphal repressor Nrg1. Aside from NRG1 downregulation, we noted repression for several ZCF members such as ZCF1, ZCF3, and FCR1, which are in some way related to filament and biofilm formation. In particular, Vandeputte et al. (2011) reported that deletion of ZCF3 significantly enhances hyphal formation. Thus, it will be useful to further investigate the effects of Rha1 on ZCF3 and the other transcriptionally influenced zinc cluster TF genes.
The induction of filamentation caused by Rha1 activation in the absence of external signals depended entirely on Brg1, while loss of Ume6 reduced, but did not eliminate, Rha1-hyperactivity-induced filamentation. The deletion of both BRG1 and UME6 together completely blocked the filamentation caused by hyperactive Rha1 under yeast growth conditions. Recent analysis of gene expression during growth in both solid and liquid conditions showed that a series of hyphal induction conditions (10% fetal bovine serum, Lee’s media, RPMI media, and Spider media) did not significantly induce or repress RHA1 expression (Azadmanesh et al. 2017). RHA1 expression has however been found to increase in cells lacking Tup1 or Nrg1 when compared with the WT, and in these strains is primarily expressed within the first 2 h after serum treatment at 37°C (Kadosh and Johnson 2005; Banerjee et al. 2008). Overall then, hyperactivation of Rha1 in the presence or absence of serum stimulation establishes an active link between Rha1 function and the Nrg1-Brg1 switch regulating chromatin structure at HAG promoters and the initiation of the hyphal developmental program.
The inactivation of RHA1 also provides evidence for a link between Rha1 and the Nrg1/Brg1-Ume6 circuit. The inactivation mutants showed limited defects in hyphal development, as filamentation was moderately delayed in serum-stimulated cells, and more significantly delayed in Spider medium induced cells. However, the rha1 ume6 double mutant was utterly defective in serum or Spider-induced hyphal development, although either mutant alone was capable of initiating hyphal formation. This suggests some redundancy in the roles of Rha1 and Ume6 in the hyphal developmental program.
Interestingly, Rha1 may function in more than just the yeast to hyphal transition. Genes involved in arginine biosynthesis were also up-regulated in cells with a hyperactivated Rha1. Previous work has suggested a linkage between hyphal development and arginine biosynthesis (Jiménez-López et al. 2013); Rha1 may thus play a role in connecting these two cellular processes. Hyperactivity of Rha1 resulted in attenuated virulence in the mouse model, with a significant change in survival curves. This may result from driving hyphal growth through Rha1 hyperactivity affecting dissemination, as evidence suggests that yeast form cells are vital for escape from the bloodstream, and adhere better than filament cells to endothelial cells (Saville et al. 2003). The result is consistent with previous reports where BRG1 overexpression or the inactivation of NRG1 attenuated virulence in systemic infections (Murad et al. 2001; Cleary et al. 2012). By contrast, the rha1Δ/Δ single mutants showed normal virulence in the mouse tail-vein injection assay even though they exhibited a minor defect in establishing abundant hyphae.
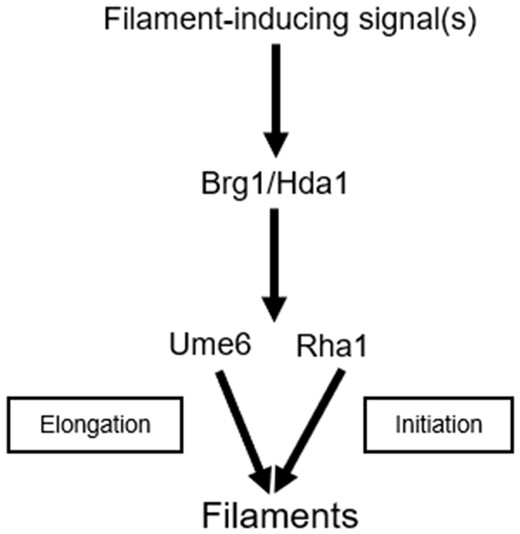
In Figure 8, we propose a genetic interaction model to highlight the relationships among Rha1, Ume6 and Brg1 in response to hyphal cues. This model proposes that Brg1/Hda1 can act through Rha1 and Ume6 after being exposed to hyphal-inducing stimuli like serum. A prior study demonstrated that constitutively expressed Ume6 corrected the hyphal growth defect in hda1 mutant cells (Lu et al. 2012), and we found that Rha1 hyperactivation bypassed the inactivation of Brg1 in serum-stimulated cells. According to this model, Rha1 and Ume6 can contribute cooperatively to hyphal formation, as we found single rha1 or ume6 mutants permit filamentation, but the double mutant fails to form hyphal cells in response to inducing cues. We suggest Rha1 and Ume6 play primarily distinct roles in filamentation; Rha1 in initiation and Ume6 in elongation. Rha1 activation uniquely induced arginine biosynthesis genes that are known to be critical for the initiation step in C. albicans filamentation (Ghosh et al. 2009), while it did not induce Ume6-triggered genes such as CDC10, RBT4, CBP1, FAV2, CDC10, PHR1, and RDI1, which may be important in elongation (Carlisle and Kadosh 2013). Key HAGs like ALS3, ECE1, HWP1, and HGC1 can be up-regulated by activation of either Rha1 or Ume6. In addition, Rha1 may also influence upstream elements, as the overlap of the transcriptional profiles and the ChIP-chip profiles suggest Brg1, Cek1, and Def1 (Eed1) are potential Rha1 target genes. Because the Rha1-GOF expression profile also contains uncharacterized TFs from the ZCF family, including ZCF4 that may play a role in Rha1-responsive filamentation, it appears there is considerable complexity in the network that still needs to be resolved.
Schematic representation of Rha1 genetic interactions during the yeast to hyphal transition. Filament generating signal(s) like serum induce the expression of Brg1 and stimulate the downstream pathways. We propose that Brg1/Hda1 directs filamentation via both Rha1, which primarily acts in initiation, and Ume6, which primarily acts in hyphal elongation. Ume6 has previously been implicated in elongation and, when activated, has been shown to bypass the requirement of Brg1/Hda1 (Lu et al. 2012). We show here that activated Rha1 in the presence of serum stimulus can similarly bypass the deletion of Brg1. We also find that although either single mutant is able to facilitate filamentation, the double rha1 ume6 mutant is unable to form hyphae. Furthermore, activation of Rha1 in a ume6 mutant generates frequent short hyphae that are inefficiently extended, consistent with a role of Ume6 in extension, while activation of Ume6 in a rha1 mutant background allows extension of all initiated filaments.
Overall, our study provides potential insight into the mechanism of hyphal development controlled by the Nrg1/Brg1 switch. Under hyphal-inducing conditions, Rha1 functions to facilitate both downregulation of the Nrg1 repressor and upregulation (directly or indirectly) of the Brg1 and Ume6 TFs required for initiation and maintenance of HAGs. This switches the cell from a repressed chromatin configuration at the HAGs controlled by Nrg1 to the activated state controlled by Brg1, and thus Rha1 represents an important new regulator of the yeast to hyphal transition critical for C. albicans pathogenicity.
Data availability
Strains and plasmids are available upon request.
Supplementary material presents Supplementary Figure S1: the effect of different stresses on strains expressing Rha1-GOF, Supplementary Figure S2: RNAseq analysis of Rha1-GOF. Supplementary Figure S3: Rha1 strains grow normally on media without arginine. Supplementary Figure S4: One copy of Rha1 restores the filamentation morphology in rha1Δ/Δ. Strains and list of primers are listed in Supplementary Tables S1 and S2. Supplementary Table S3 represents the GO term analysis of Rha1-GOF upregulated (95 genes) and downregulated genes (34 genes). Supplementary Table S4 shows ChIP-chip data and Transcription profiling data of Rha1GOF. Supplementary files are available at figshare: https://doi.org/10.25386/genetics.14600349. RNA sequencing data are accessible through GEO Series accession number GSE143825.
Acknowledgments
The authors thank all members of the M. Hallett lab for helpful discussions, and M.W. lab members for their valuable contribution during this study. This study is dedicated to Rebecca Schillig (deceased), who originally discovered orf19.1604 (RHA1) as a regulator of filamentous growth of C. albicans during her PhD studies in J.M.’s lab.
Funding
We want to acknowledge funding support from the Natural Sciences and Engineering Research Council of Canada Discovery Grant RGPIN/4799 (M.W.), Natural Sciences and Engineering Research Council of Canada ,Canada Research Chair 950-228957 awards (M.W.), the Deutsche Forschungsgemeinschaft (DFG grant MO 846/7) (J.M.) and National Nature Science Foundation of China funding No. 82072261 (J.F.).
Conflicts of interest
The authors declare that there is no conflict of interest.