-
PDF
- Split View
-
Views
-
Cite
Cite
Ziyi Liu, Lifen Jiang, Chaoyi Li, Chengang Li, Jingqun Yang, Jianjun Yu, Renbo Mao, Yi Rao, LKB1 is physiologically required for sleep from Drosophila melanogaster to the Mus musculus, Genetics, Volume 221, Issue 3, July 2022, iyac082, https://doi.org/10.1093/genetics/iyac082
Close - Share Icon Share
Abstract
LKB1 is known as a master kinase for 14 kinases related to the adenosine monophosphate (AMP)-activated protein kinase (AMPK). Two of them (SIK3 and AMPKa) have previously been implicated in sleep regulation. We generated loss-of-function (LOF) mutants for Lkb1 in both Drosophila and mice. Sleep, but not circadian rhythms, was reduced in Lkb1-mutant flies and in flies with neuronal deletion of Lkb1. Genetic interactions between Lkb1 and AMPK T184A mutants in Drosophila sleep or those between Lkb1 and SIK3 T196A mutants in Drosophila viability have been observed. Sleep was reduced in mice after virally mediated reduction of Lkb1 in the brain. Electroencephalography (EEG) analysis showed that non-rapid eye movement (NREM) sleep and sleep need were both reduced in Lkb1-mutant mice. These results indicate that LKB1 plays a physiological role in sleep regulation conserved from flies to mice.
Introduction
Human Peutz-Jeghers syndrome (PJS) (Peutz 1921; Jeghers et al. 1949) is an autosomal dominant disorder with gastrointestinal (GI) polyps and increased cancer risk of multiple tissues (Tomlinson and Houlston 1997; Westerman et al. 1999). The gene mutated in, and responsible for, PJS encodes the liver kinase B1 (LKB1, also known as STK11) (Hemminki et al. 1997; 1998; Jenne et al. 1998). Lkb1 is thus a tumor suppressor gene, mutated in multiple cancers, especially the GI (Mehenni et al. 1998; Bardeesy et al. 2002; Jishage et al. 2002; Miyoshi et al. 2002; Hearle et al. 2006) and lung adenocarcinoma (Sanchez-Cespedes et al. 2002; Carretero et al. 2004; Ji et al. 2007; Matsumoto et al. 2007; Gill et al. 2011; Skoulidis et al. 2015), cervical cancer (Wingo et al. 2009), ovarian cancer (Tanwar et al. 2014), breast cancer (Shen et al. 2002; Hearle et al. 2006; Sengupta et al. 2017), pancreatic cancer (Morton et al. 2010), and melanoma (Guldberg et al. 1999; Rowan et al. 1999).
LKB1 phosphorylates threonine 172 (T172) of the α subunit of adenosine monophosphate (AMP)-activated protein kinase (AMPKα) (Hawley et al. 2003; Hong et al. 2003; Sutherland et al. 2003; Woods et al. 2003; Lizcano et al. 2004; Shaw et al. 2004, 2005; Sakamoto et al. 2005), and positively regulates the activity of AMPK.
AMPK is a well-known kinase (Beg et al. 1973; Carlson and Kim 1973; Ingebritsen et al. 1978; Yeh and Kim 1980; Ferrer et al. 1985; Carling et al. 1987, 1989; Munday et al. 1988) with important physiological and pathological roles (Hardie 2014; Lopez and Dieguez 2014; Hardie et al. 2016; Herzig and Shaw 2018). The α, β, and γ subunits of AMPK form a heterotrimeric complex (Davies et al. 1994; Mitchelhill et al. 1994; Michell et al. 1996). The catalytic α subunit is regulated by phosphorylation at T172 of AMPKα2 or T183 of AMPKα1 (Hawley et al. 1996).
There are 12 additional mammalian AMPK-related kinases (ARKs), similar to the α subunit of AMPK, all regulated at the site equivalent to AMPK-T172 (Lizcano et al. 2004). LKB1 and its associated proteins STE20-related adaptor (STRAD) and mouse protein 25 (MO25) have been reported to phosphorylate all 14 ARKs (Lizcano et al. 2004), making LKB1 a master kinase for ARKs (Lizcano et al. 2004; Alessi et al. 2006; Shackelford and Shaw 2009). We have recently found that more than 20 kinases in the STE20 family of mammalian serine-threonine kinases could phosphorylate ARKs in vitro, though the physiological roles of STE20 kinases in ARK phosphorylation remain unknown (Liu, Wang, Cui, Gao, et al. 2022; Liu, Wang, Cui, et al. 2022).
Some ARKs have been reported to regulate sleep. In mice, inhibitors of AMPK were found to decrease sleep, whereas activators of AMPK were found to increase sleep (Chikahisa et al. 2009). In flies, knockdown of AMPKβ in neurons decreased the total amount of sleep and resulted in fragmented sleep (Nagy et al. 2018). Knockdown of AMPKα in a specific pair of neurons suppressed sleep (Yurgel et al. 2019).
Studies in mice have shown that sleep was increased in gain-of-function (GOF) mutations in the salt inducible kinase (SIK) 3 (Funato et al. 2016), and sleep need was reduced in GOF mutants of SIK 1, 2, and 3 (Funato et al. 2016; Honda et al. 2018; Park et al. 2020). Sleep was also decreased when SIK3 was downregulated in flies (Funato et al. 2016). Here we investigated the functional role of LKB1 in regulating sleep in flies and mice.
Materials and methods
Fly lines and rearing conditions
Flies were reared on standard corn meal at 25°C, 60% humidity and kept in 12 hours (h) light/12 h dark (LD) conditions. 57C10-Gal4, nos-phiC31, hs-Cre on X were from the Bloomington Stock Center. vas-Cas9 was a gift from Dr J. Ni (Tsinghua University, Beijing). Upstream activation sequence (UAS)-Cas9, UAS-Ampk, UAS-Ampk-T184A, UAS-Ampk-T184E, Sik3-flag, Sik3–T196A-flag, and Sik3-T196E-flag flies were from our laboratory.
Flies used in behavioral assays were backcrossed into our isogenized Canton S background for 7 generations. All results of sleep analysis in this paper were obtained from female flies.
Generation of KO, KI, and transgenic lines
Total RNA was extracted from isoCS by TRIzol reagent (Invitrogen). Using the PrimeScript II 1st Strand cDNA Synthesis Kit (Takara), we reverse-transcribed the extracted mRNA into cDNA. The UAS-Lkb1 flies was constructed by inserting the coding sequence (CDS) of CG9374 amplified from cDNA into the pACU2 plasmid (a gift from the Jan Lab at UCSF) (Han et al. 2011) before being inserted into the attP40 site.
The UAS-Lkb1-sgRNA construct was designed by inserting the sgRNAs into pMt: sgRNA3XEF vectors based on pACU2, with rice tRNA separating the different sgRNAs. CRISPR-Gold website was used to design 3 sgRNAs of Lkb1 (Supplementary Fig. 3) (Chu et al. 2016; Poe et al. 2019). The construct was inserted into the attP40 site.
KO and KI lines were generated as described previously (Deng et al. 2019). Knockout flies were generated with the CRISPR/Cas9 system. Two different sgRNAs were constructed with U6b-sgRNA plasmids. The 5′ homologous arm and the 3′ homologous arm of ∼2 kb amplified from the wt fly genome were inserted into a pBSK plasmid for homologous recombination repair. The cassette of attP-3P3-RFP was introduced in the middle. sgRNA plasmids and the donor plasmids were injected into vas-Cas9 embryos to introduce attP-3P3-RFP into the genome at the region of interest and replaced it by homologous recombination. 3P3-RFP served as a marker to screen for the correct flies. Primers across the homologous arms were designed to verify the sequences by PCR and DNA sequencing. attP site was introduced into the genome with 3P3-RFP-LoxP. For KI files, the nos-phiC31 virgin females were first crossed with knock-out males and the pBSK plasmid inserted with attB-T2A-Gal4-miniwhite-LoxP cassette was injected into the female embryos. Miniwhite serves as a marker to screen for the correct flies, which could be excised by the Cre/LoxP system. Primers were designed to verify the sequence by PCR and DNA sequencing.
Quantitative PCR
Total RNA was extracted from 30 flies of 5–7 days old by TRIzol reagent (Invitrogen). The genomic template was removed using DNase (Takara). cDNA was reverse-transcribed using Takara’s PrimeScript II 1st Strand cDNA synthesis kit (Takara). Quantitative PCR was carried out with TransStart Top Green qPCR SuperMix kit (TransGen) in the Bio-Rad PCR system (CFX96 Touch Deep Well). The sequences of primers used to detect Lkb1 and RP49(endogenous control) mRNA are
Lkb1-F: 5′-GCCGTCAAGATCCTGACTA-3′
Lkb1-R: 5′-CTCCGCTGGACCAGATG-3′
Rp49-F: 5′-CGACGCTTCAAGGGACAGTATC-3′
Rp49-R: 5′-TCCGACCAGGTTACAAGAACTCTC-3′
Drosophila sleep analysis
Drosophila sleep analysis was performed as described previously (Qian et al. 2017; Dai et al. 2019). 5–7 days old flies were placed in a 65 mm × 5 mm clear glass tube with one end containing food and the other end plugging with cotton. All flies were recorded by video-cameras. Before sleep measurement, flies were entrained to an LD cycle at 25°C, 60% humidity for at least 2 days, and infrared LED light was used to ensure constant illumination when lights off. Immobility longer than 5 min was defined as one sleep event (Hendricks et al. 2000; Shaw et al. 2000). Information of fly location was tracked and sleep parameters were analyzed using Matlab (Mathworks), from which dead flies were removed. Sleep duration, sleep bout duration, sleep bout number, and sleep latency for each LD were analyzed. Each experiment was repeated at least 3 times.
Drosophila circadian analysis
Flies were reared and recorded in the same condition as sleep assay as described in papers from our laboratory (Qian et al. 2017; Dai et al. 2021), except that the condition was constant darkness. Six to 8 days activity was measured and calculated in ActogramJ (Klarsfeld et al. 2003). Rhythmic strength, power and period were calculated by Chi-square method.
Immunoblot analysis
Mouse brains were quickly dissected and washed with phosphate buffer saline on ice. Lysis buffer (20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, freshly supplemented with a protease and phosphatase inhibitors cocktail) were used to homogenize brains by homogenizer (Wiggens, D-500 pro) at 4°C. Brain homogenates were centrifuged at 14,000 revolution per minutes for 15 min at 4°C. The supernatant was transferred to a new microtube and quantified with bicinchoninic acid assay (Thermo Fisher, 23225). The supernatant was analyzed by SDS-PAGE and proteins were transferred to a nitrocellulose membrane (GE Healthcare, #BA85). Membranes were incubated for 1 h in a blocking solution (tris-buffered saline containing 0.1% Tween-20, 5% milk). Primary antibodies were anti-LKB1 (cell signaling, #3047) and anti-ACTIN (Santa Cruz, sc-8342).
Retro-orbital injection in mice
Mice were reared at controlled temperature and humidity conditions with 12 h light/12 h dark cycle. Food and water were provided ad libitum. Lkb1fl/fl mice were from the Jackson Laboratory (JAX #014143). They contained loxP sites flanking exons 3–6 of Lkb1 gene (Nakada et al. 2010). adeno-associated viral (AAV)-PHP.eB-hSyn-Cre-green fluorescent protein (GFP) and AAV-PHP.eB-hSyn-GFP virus were from Chinese Institute for Brain Research, Beijing. All results of sleep analysis in this paper were obtained from female mice.
Total 0.2 ml/10 g avertin was injected intraperitoneally into the mice for anesthetization. Rodent eyes were protruded by gentle downward pressure to the skin on the dorsal and ventral sides of the eye. The operator inserted the needle beveled downward into the retro-orbital sinus at the medial corner of the eye (Yardeni et al. 2011). The AAV-PHP.eB virus was injected for whole brain infection (Chan et al. 2017).
Mouse sleep analysis
Mouse sleep analysis was described in a previous article from our laboratory (Zhang et al. 2018). Eight-week-old mice were selected for retro-orbital injection. One week after viral injection, EEG and EMG electrode implantation procedures were performed. Mice were allowed to recover for more than 5 days individually and placed in a recording cage and tethered to an omni-directional arm (RWD Life Science Inc.) with connection cable for 2 days of habituation before recording. EEG and EMG data were recorded with custom software at a sampling frequency of 200 Hz for 2 consecutive days to analyze sleep/wake behavior under baseline conditions. The recording chamber was maintained at 12 h LD cycle and controlled temperature (24–25°C). EEG/EMG data were initially processed by Accusleep (Barger et al. 2019) before manual correction in SleepSign to improve accuracy. WAKE was scored as high amplitude and variable EMG and fast and low amplitude EEG. Nonrapid eye movement (NREM) was scored as high amplitude δ (1–4 Hz) frequency EEG and low EMG tonus. REM was scored as a complete silent of EMG signals and low amplitude high frequency θ (6–9 Hz)-dominated EEG signal.
For power spectrum analysis, EEG was subjected to fast Fourier transform analysis with a Hamming window method by SleepSign, yielding power spectra between 0 and 25 Hz with a 0.39 Hz bin resolution. Epochs containing movement artifacts were marked, included in sleep duration analysis but excluded from the power spectra analysis. Power spectra for each vigilance state represents the mean power distribution of this state during a 24-h baseline recording. The δ-power density of NREMs per hour represents the average of δ-power density as a percentage of δ-band power (1–4 Hz) to total power (0–25 Hz) for each NREM epoch contained in an hour.
Statistics
All statistical analyses were performed with Prism 7 (GraphPad Software). Differences in means between samples larger than 2 groups were analyzed using ordinary 1-way ANOVA. Unpaired t test was used for 2 groups comparison. Power spectrum between different lines was compared by 2-way ANOVA followed by Turkey’s multiple comparisons test. n.s. denotes P > 0.05; *P < 0.05; **P < 0.01; and ***P < 0.001 for all statistical results in this paper.
Results
Sleep phenotypes of Drosophila Lkb1 mutants
Null mutants for Lkb1 are lethal in Drosophila (Martin and St Johnston 2003). We had generated a Lkb1 knockout (“lkb1T1”) line (Supplementary Figs. 1a and 3b) and found that lkb1T1/T1 mutation was lethal in the pupa stage. The level of Lkb1 mRNA was reduced in the heterozygous lkb1T1/+ flies (Supplementary Fig. 1b). We then tested whether the heterozygous lkb1T1 had any phenotype in sleep using flies kept in 12 hours (h) light/12 h dark (LD) cycles (Supplementary Fig. 1c). While lkb1T1/+ flies were not significantly different from the wild-type (wt) flies in sleep bout numbers (Supplementary Fig. 1e), or daytime sleep duration (Supplementary Fig. 1d), daytime sleep bout duration (Supplementary Fig. 1f), lkb1T1/+ flies showed significantly lower nighttime sleep duration (Supplementary Fig. 1d), nighttime sleep bout duration (Supplementary Fig. 1f), and longer latency to sleep (Supplementary Fig. 1g). Thus, there was dosage-sensitive physiological requirement of Lkb1 in nighttime sleep.
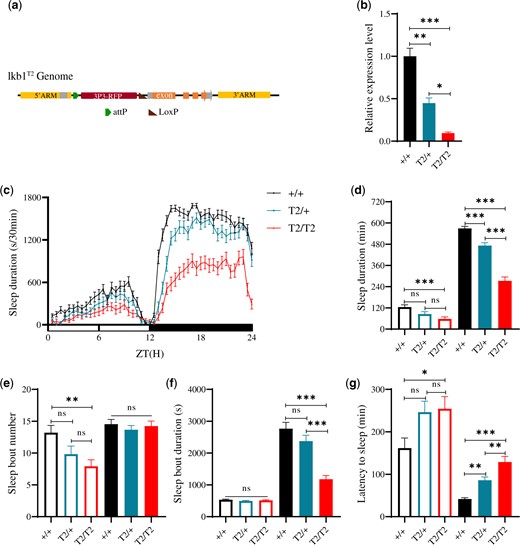
We tried to, and succeeded in, generating lkb1T2, a hypomorphic mutation for Lkb1 in flies (Fig. 1a;Supplementary Fig. 3, a and b). Lkb1 mRNA was significantly reduced in lkb1T2/+ and lkb1T2/T2 flies (Fig. 1b). During the day, lkb1T2/T2 flies were not significantly different from the lkb1T2/+ and wt flies in sleep duration (Fig. 1, c and d), sleep bout number (Fig. 1e), sleep bout duration (Fig. 1f), or latency to sleep (Fig. 1g). During the night, not only lkb1T2/T2 flies showed significantly reduced sleep duration (Fig. 1, c and d), highly reduced sleep bout duration (Fig. 1f) and highly increased latency (Fig. 1g) than the wt flies, but also the heterozygous lkb1T2/+ flies were significantly different from the wt flies in all these parameters (Fig. 1, c–g), indicating a dosage sensitive requirement for Lkb1.
Sleep phenotypes of Lkb1 knock-down mutant flies. a) A diagram illustrating the Lkb1 insertion mutant lkb1T2. b) Relative Lkb1 mRNA levels in lkb1T2/T2 (red), lkb1T2/+ (blue), and wt (black) flies. c) Sleep profiles of lkb1T2/T2 (red, n = 42), lkb1T2/+ (blue, n = 44), and wt (black, n = 44) flies in a 12 h light/12 h dark (LD) cycle. Statistical analysis of sleep duration, sleep bout number, sleep bout duration, and latency to sleep in lkb1T2/T2 (red, n = 42), lkb1T2/+ (blue, n = 44), and wt (black, n = 44) flies. Open bars denote daytime, filled bars denote nighttime. d) Sleep duration. Nighttime sleep durations of lkb1T2/T2 mutants were significantly less than those in lkb1T2/+ and wt flies. e) Sleep bout number. Daytime sleep bout number of lkb1T2/T2 mutants was less than that of wt flies. f) Sleep bout duration. Nighttime sleep bout duration of lkb1T2/T2 mutants was significantly less than those of lkb1T2/+ and wt flies. g) Latency to sleep. Latency to sleep after light-off of lkb1T2/T2 mutants was significantly prolonged than lkb1T2/+ and wt flies. One-way ANOVA was used. n.s. denotes P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent standard error of the mean (SEM).
We examined the phenotypes of lkb1T1/T2. Consistent with the lkb1T2/T2, the mRNA levels of Lkb1 were significantly reduced in lkb1T1/T2 compared with wt, lkb1T1/+ and lkb1T2/+, and even lower than that in lkb1T2/T2 (Supplementary Fig. 2a).
The sleep phenotype in lkb1T1/T2 flies was also consistent with lkb1T2/T2, with highly reduced nighttime sleep duration (Supplementary Fig. 2, b and c), highly reduced sleep bout duration (Supplementary Fig. 2e) and highly increased latency to sleep (Supplementary Fig. 2f), when compared with wt, lkb1T1/+, and lkb1T2/+ flies.
Results of sleep analysis of lkb1T1/+, lkb1T2/+, lkb1T2/T2, and lkb1T1/T2 mutant flies all consistently support that Lkb1 plays a physiological role in promoting sleep.
Rescue of sleep phenotypes by Lkb1 in flies
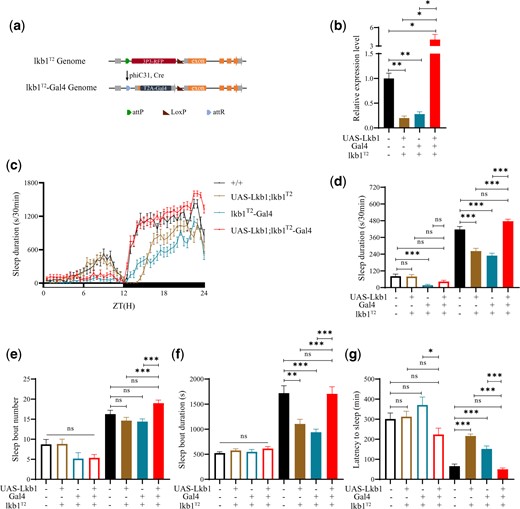
We inserted the sequence of the yeast transcription factor Gal4 into the lkb1T2 mutant flies, flanking the lkb1 promoter, and obtained lkb1T2-Gal4 flies (Fig. 2a). We also generated UAS-Lkb1 flies in which the Lkb1 CDS was expressed under the control of the UAS (Brand and Perrimon 1993). Because Gal4 protein binds to the UAS, the expression of Lkb1 in flies resulting from the crosses between lkb1T2-Gal4 flies and UAS-Lkb1 flies would be under the control of the endogenous Lkb1 promoter. Indeed, expression of Lkb1 mRNA was restored when lkb1T2-Gal4 and UAS-Lkb1 were present in the same flies (Fig. 2b), whereas Lkb1 mRNA was less in UAS-Lkb1; lkb1T2/T2 mutant flies, and lkb1T2-Gal4/lkb1T2-Gal4 flies than that in the wt. UAS-Lkb1 alone could not restore Lkb1 mRNA expression level to that in wt flies (Fig. 2b).
Rescue of sleep loss in lkb1T2/T2 by Lkb1. a) A diagram of lkb1T2-Gal4: a cDNA for the yeast Gal4 gene inserted in the lkb1T2 knockdown mutants. b) Relative Lkb1 mRNA levels in lkb1T2-Gal4 (blue), UAS-Lkb1; lkb1T2-Gal4 (red), UAS-Lkb1; lkb1T2 (yellow), and wt (black) flies. In lkb1T2-Gal4 homozygous flies, UAS-Lkb1 cDNA driven by Gal4 to rescue sleep phenotypes of lkb1 knockdown mutants. c) Sleep profiles of UAS-Lkb1; lkb1T2-Gal4 (red, n = 45), UAS-Lkb1; lkb1T2 (yellow, n = 47), lkb1T2-Gal4 (blue, n = 46), and wt (black, n = 36) flies. Statistical analysis of sleep duration, sleep bout number, sleep bout duration and latency to sleep in UAS-Lkb1; lkb1T2-Gal4 (red, n = 45), UAS-Lkb1; lkb1T2 (yellow, n = 47), lkb1T2-Gal4 (blue, n = 46), and wt (black, n = 36) flies. Open bars denote daytime, filled bars nighttime. d) Sleep duration. Nighttime sleep duration of UAS-Lkb1; lkb1T2-Gal4 was similar to that of wt mutants, both significantly higher than UAS-Lkb1; lkb1T2 and lkb1T2/T2-Gal4 flies. e) Sleep bout number. Nighttime sleep bout number of UAS-Lkb1; lkb1T2-Gal4 was similar to the wt but significantly higher than UAS-Lkb1; lkb1T2 and lkb1T2-Gal4 flies. f) Sleep bout duration. Nighttime sleep bout duration of UAS-Lkb1; lkb1T2-Gal4 was similar to the wt but significantly higher than UAS-Lkb1; lkb1T2 and lkb1T2-Gal4 flies. g) Latency to sleep. Latency after light-off of UAS-Lkb1; lkb1T2-Gal4 was similar to the wt but significantly shorter than UAS-Lkb1; lkb1T2 and lkb1T2-Gal4 flies. One-way ANOVA was used. n.s. denotes P > 0.05, * P < 0.05, ** P < 0.01, ***P < 0.001. Error bars represent SEM.
Both daytime and nighttime sleep durations were less in lkb1T2-Gal4/lkb1T2-Gal4 flies than those in wt flies (Fig. 2c). Introduction of UAS-Lkb1 in lkb1T2/T2 flies or lkb1T2-Gal4 alone could not restore sleep. When both lkb1T2-Gal4 and UAS-Lkb1 were present, nighttime sleep durations were restored (Fig. 2d). Nighttime sleep bout number, nighttime sleep bout duration, and nighttime latency were restored when both lkb1T2-Gal4 and UAS-Lkb1 were present, but not when lkb1T2-Gal4 or UAS-Lkb1 alone was present (Fig. 2, e–g).
These results support that the sleep phenotypes of lkb1T2/T2 were attributable to the reduction of Lkb1 mRNA expression in these flies.
Sleep phenotypes of flies carrying neuronal deletion of the Lkb1 gene
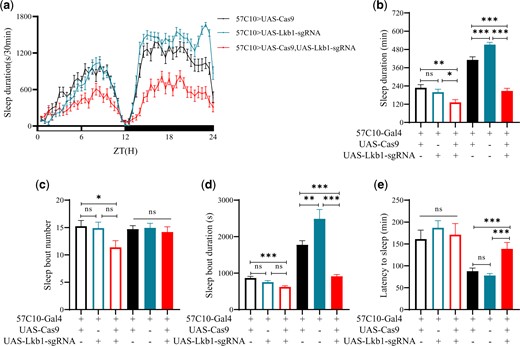
To determine whether Lkb1 functions in neurons, we used the CRISPR-Cas9 system to delete Lkb1 from neurons specifically (Supplementary Fig. 4). A pan-neuronal Gal4 driver (57C10-Gal4) was used to control the expression of small guide RNA (sgRNA) targetting Lkb1 in neurons. Compared with 57C10-Gal4>UAS-Cas9 alone or 57C10-Gal4>UAS-Lkb1-sgRNA alone, when both UAS-Cas9 and UAS-Lkb1-sgRNA were present in flies, nighttime sleep duration (Fig. 3b) and nighttime sleep bout duration (Fig. 3d) were significantly reduced and nighttime sleep latency significantly lengthened (Fig. 3e). Daytime sleep duration, bout number, bout duration and latency were not significantly affected by neuronal gene targeting of Lkb1 (Fig. 3, b–e).
Sleep phenotypes of mutants from whose neurons Lkb1 was targeted. a) Sleep profiles of UAS-Lkb1-sgRNA/57C10-Gal4;+/UAS-Cas9 (red, n = 44), UAS-Lkb1-sgRNA/57C10-Gal4 (blue, n = 41), and 57C10-Gal4/+;+/UAS-Cas9 (black, n = 45) flies. Statistical analysis of sleep duration, sleep bout number, sleep bout duration, and latency to sleep in UAS-Lkb1-sgRNA/57C10-Gal4;+/UAS-Cas9 (red, n = 44), UAS-Lkb1-sgRNA/57C10-Gal4 (blue, n = 41), and 57C10-Gal4/+;+/UAS-Cas9 (black, n = 45) flies. Open bars denote daytime, filled bars nighttime. b) Sleep duration. Daytime and nighttime sleep duration of UAS-Lkb1-sgRNA/57C10-Gal4;+/UAS-Cas9 was significantly less than those of UAS-Lkb1-sgRNA/57C10-Gal4 and 57C10-Gal4/+;+/UAS-Cas9 flies. c) Sleep bout number. Daytime and nighttime sleep bout numbers of UAS-Lkb1-sgRNA/57C10-Gal4;+/UAS-Cas9 was not significantly from those of UAS-Lkb1-sgRNA/57C10-Gal4 and 57C10-Gal4/+;+/UAS-Cas9 flies. d) Sleep bout duration. Nighttime sleep bout duration of UAS-Lkb1-sgRNA/57C10-Gal4;+/UAS-Cas9 was significantly less than that of UAS-Lkb1-sgRNA/57C10-Gal4 and 57C10-Gal4/+;+/UAS-Cas9 flies. e) Latency to sleep. Latency to sleep after light-off of UAS-Lkb1-sgRNA/57C10-Gal4;+/UAS-Cas9 was longer than that of 57C10-Gal4/+;+/UAS-Cas9 which was not significantly different from that of UAS-Lkb1-sgRNA/57C10-Gal4 flies. One-way ANOVA was used. n.s. denotes P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent SEM.
We also investigated any potential effect that overexpression of Lkb1 in neurons might cause (Supplementary Fig. 5a). We detected no phenotype resulting from neuronal overexpression of Lkb1 on daytime and nighttime sleep duration, sleep bout number, sleep bout duration, or latency (Supplementary Fig. 5, b–f).
In all 3 series of experiments (Figs. 1–3), nighttime sleep phenotypes were more obvious than daytime sleep phenotypes. These results strongly indicate that Lkb1 expression in neurons are required physiologically for sleep, especially nighttime sleep.
Genetic interactions between Lkb1 and Ampk or Sik3 in flies
To examine potential genetic interactions of Lkb1 with either Ampk or Sik3, we combined the loss-of-function (LOF) Lkb1 mutation lkb1T2 with specific point mutations in either Ampk or Sik3.
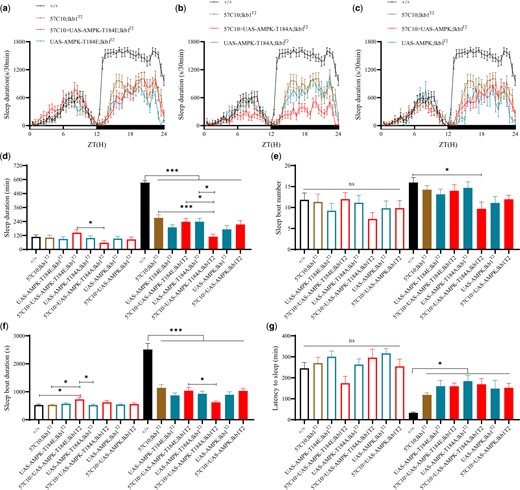
The regulatory site T184 in Drosophila AMPK and T196 in Drosophila SIK3 were equivalent to T172 of mammalian AMPK2 and T221 of mammalian SIK3, important for their activities. When the endogenous T184 in fly AMPK was mutated to alanine (A) or glutamic acid (E), flies were lethal. We therefore introduced T184A and T184E mutations into an Ampk transgene whose expression was controlled by UAS. We introduced UAS-Ampk, UAS-Ampk-T184A, and UAS-Ampk-T184E into lkb1T2/T2 flies and used a pan-neuronal driver to express them in neurons (Fig. 4). Neuronal overexpression of Ampk-T184E (Fig. 4a) and UAS-Ampk (Fig. 4c) in lkb1T2 flies did not significantly change the sleep phenotypes of lkb1T2/T2 flies, but neuronal overexpression of UAS-Ampk-T184A (Fig. 4b) in lkb1T2/T2 flies further decreased nighttime sleep duration.
Genetic interactions between lkb1 and ampk. a) Sleep profiles of UAS-Ampk-T184E/57C10; lkb1T2 (red, n = 19), 57C10; lkb1T2 (yellow, n = 24), UAS-Ampk-T184E; lkb1T2 (blue, n = 22) and wt (black, n = 24) flies. b) Sleep profiles of UAS-Ampk-T184A/57C10; lkb1T2 (red, n = 24), 57C10; lkb1T2 (yellow, n = 24), UAS-Ampk-T184A; lkb1T2 (blue, n = 23) and wt (black, n = 24) flies. c) Sleep profiles of UAS-Ampk/57C10; lkb1T2 (red, n = 24), 57C10; lkb1T2 (yellow, n = 24), UAS-Ampk; lkb1T2 (blue, n = 11) and wt (black, n = 24) flies. (d–g) Statistical analysis of sleep duration, sleep bout number, sleep bout duration, and latency to sleep in wt (black, n = 24), 57C10; lkb1T2 (yellow, n = 24), UAS-Ampk-T184E; lkb1T2 (blue, n = 22), UAS-Ampk-T184E/57C10; lkb1T2 (red, n = 19), UAS-Ampk-T184A; lkb1T2 (blue, n = 23), UAS-Ampk-T184A/57C10; lkb1T2 (red, n = 24), UAS-Ampk; lkb1T2 (blue, n = 11), and UAS-Ampk/57C10; lkb1T2 (red, n = 24) flies. Open bars denote daytime, filled bars nighttime; n.s. not shown. One-way ANOVA was used. n.s. denotes P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent SEM.
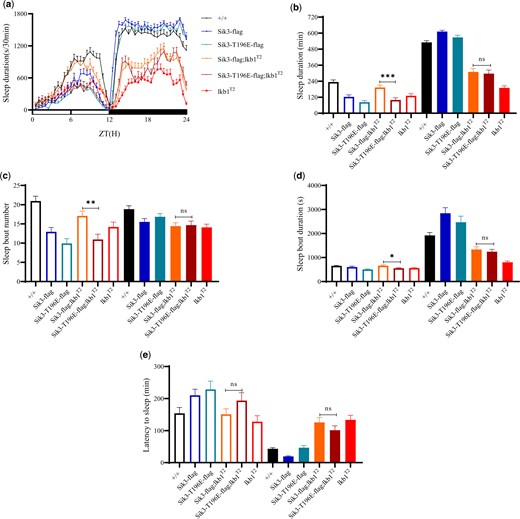
Point mutations of Sik3-flag, Sik3-T196A-flag, and Sik3-T196E-flag were constructed in Drosophila. When the endogenous T196 in Sik3 was mutated to A or E, we could get homozygous flies. Upon crossing to lkb1T2/T2, Sik3-T196A-flag; lkb1T2/T2 were homozygous lethal. The cross of Sik3-T196E-flag into the lkb1T2/T2 background generated viable flies, with no detectable change in sleep (Fig. 5).
Genetic interactions between lkb1 and sik3. a) Sleep profiles of lkb1T2 (red, n = 45), Sik3-T196E-flag; lkb1T2 (dark red, n = 46), Sik3-flag; lkb1T2 (orange, n = 48), Sik3-T196E-flag (blue, n = 45), Sik3-flag (dark blue, n = 44) and wt (black, n = 46) flies. (b–e) Statistical analysis of sleep duration, sleep bout number, sleep bout duration, and latency to sleep in wt (black, n = 46), Sik3-flag (dark blue, n = 44), Sik3-T196E-flag (blue, n = 45), Sik3-flag; lkb1T2 (orange, n = 48), Sik3-T196E-flag; lkb1T2 (dark red, n = 46), and lkb1T2 (red, n = 45) flies. Open bars denote daytime, filled bars nighttime. Statistics for groups Sik3-flag; lkb1T2 and Sik3-T196E-flag; lkb1T2 were presented. One-way ANOVA was used. n.s. denotes P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent SEM.
Allele-specific genetic interactions between Lkb1 and Ampk in sleep, or between Lkb1 and Sik3 in viability, suggest, but do not prove, regulatory relationships between Lkb1 and Ampk in sleep or Sik3 in viability.
Circadian rhythm in Lkb1 mutant flies
The transcription factor differentiated embryo-chondrocyte 1 regulates circadian rhythm and can negatively regulate the transcription of Lkb1 and subsequently reduce AMPK activity (Sato et al. 2015).
We tested whether the circadian rhythm was affected in Lkb1 mutant flies. Lkb1 mutant flies were not different from wt flies in period length (Supplementary Fig. 7b). Relative rhythmic power was increased in lkb1T2/+ and lkb1T2/T2 mutants than wt flies (Supplementary Fig. 7).
Sleep phenotypes in Lkb1 conditional knockout mice
To investigate potential involvement of Lkb1 in regulating sleep of mammalian animals, we obtained Lkb1fl/fl mice in which the loxP sites flanked exons 3–6 of the Lkb1 gene (Nakada et al. 2010). To delete the Lkb1 gene from these mice, we injected AAV constructs expressing either the Cre recombinase together with the GFP or GFP alone to infect the mouse brain. Cre-GFP or GFP was under the control of a neuronal specific promoter human Synapsin I (hSyn) (in AAV-PHP.eB-hSyn-Cre-GFP or AAV-PHP.eB-hSyn-GFP).
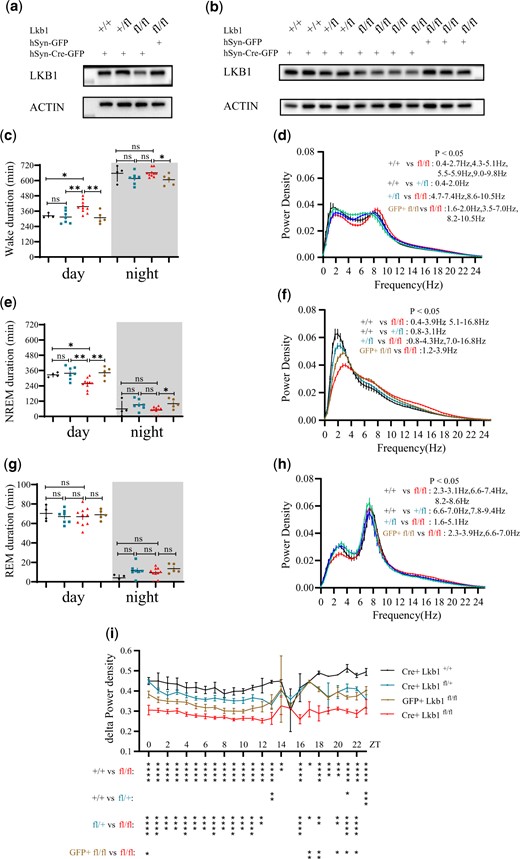
We analyzed the expression of LKB1 protein in mice (Fig. 6, a and b). Injection of Cre-GFP expressing virus into wt or Lkb1fl/+ mice did not reduce LKB1 protein expression in the brain. Neither did injection of only GFP expressing virus into Lkb1fl/fl mice. This conclusion was reached by examination of either several mouse brains combined (Fig. 6a), or individual mouse brains (Fig. 6b).
Sleep phenotypes of lkb1 conditional knockout mice. a) Levels of LKB1 protein from Lkb1fl/fl mice injected with AAV-hSyn-Cre-GFP virus (Cre+ Lkb1fl/fl, n = 4), Lkb1fl/fl mice injected with AAV-hSyn-GFP virus (GFP+ Lkb1fl/fl, n = 3), Lkb1fl/+ mice injected with AAV-hSyn-Cre-GFP virus (Cre+ Lkb1fl/+, n = 2) and Lkb1+/+ mice injected with AAV-hSyn-Cre-GFP virus (Cre+ Lkb1+/+, n = 2). These mice were among those used for EEG recording and analysis. b) Levels of LKB1 protein in individual mice (genotypes labeled: Cre+ Lkb1fl/fl, GFP+ Lkb1fl/fl, Cre+ Lkb1fl/+, and Cre+ Lkb1+/+). These mice were the same mice as those in (a) but presented individually. Statistical analysis of wake duration, NREM duration and REM duration in Cre+ Lkb1fl/fl (red, n = 10), GFP+ Lkb1fl/fl (yellow, n = 5), Cre+ Lkb1fl/+ (blue, n = 7), and Cre+ Lkb1+/+ (black, n = 4) mice in a 12:12 LD cycle. White background denotes daytime, gray background nighttime. c) Wake duration. Daytime wake duration of Cre+ Lkb1fl/fl mice was higher than those of GFP+ Lkb1fl/fl, Cre+ Lkb1fl/+, or Cre+ Lkb1+/+ mice. Night-time wake duration of Cre+ Lkb1fl/fl mice was higher than that of GFP+ Lkb1fl/fl mice. e) NREM duration. Daytime NREM duration of Cre+ Lkb1fl/fl mice was lower than those of GFP+ Lkb1fl/fl, Cre+ Lkb1fl/+, and Cre+ Lkb1+/+ mice. Night-time NREM duration of Cre+ Lkb1fl/fl mice was lower than that of GFP+ Lkb1fl/fl mice. g) REM duration. Daytime and nighttime REM durations of Cre+ Lkb1fl/fl mice was not significantly different from those of GFP+ Lkb1fl/fl, Cre+ Lkb1fl/+, and Cre+ Lkb1+/+ mice. EEG power spectrum of (d) WAKE, (f) NREM, and (h) REM states in Cre+ Lkb1fl/fl (red, n = 8), GFP+ Lkb1fl/fl (yellow, n = 5), Cre+ Lkb1fl/+ (blue, n = 6), and Cre+ Lkb1+/+ (black, n = 4) mice. (i) NREM δ-power density of Cre+ Lkb1fl/fl (red, n = 8), GFP+ Lkb1fl/fl (yellow, n = 5), Cre+ Lkb1fl/+ (blue, n = 6), and Cre+ Lkb1+/+ (black, n = 4) mice. One-way ANOVA was used in (c, e, g) for comparison of Cre+ Lkb1fl/fl, Cre+ Lkb1fl/+, and Cre+ Lkb1+/+ mice. Unpaired t test was used in (c, e, g) for comparison of Cre+ Lkb1fl/fl, and GFP+ Lkb1fl/fl mice. Two-way ANOVA followed by Turkey’s multiple comparisons test was used in (d, f, h, i). n.s. denotes P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent SEM.
Functionally, only when the Cre-GFP expressing virus was injected into Lkb1fl/fl mice, wake duration was significantly increased during daytime (Fig. 6c;Supplementary Fig. 6a), NREM sleep duration was significantly decreased during daytime (Fig. 6e;Supplementary Fig. 6b). Controls (Cre-GFP injection into wt or Lkb1fl/+ mice, GFP injection into Lkb1fl/fl mice) did not significantly changed any sleep phenotypes.
REM sleep duration was not significantly affected by Cre-GFP injection into Lkb1fl/fl mice (Fig. 6g;Supplementary Fig. 6c).
Power density in the 1–4 Hz range (δ power density) of NREM is a commonly accepted indicator of sleep need (Borbely et al. 1981; Borbely 1982; Daan et al. 1984; Tobler and Borbely 1986; Dijk et al. 1987; Werth et al. 1996; Franken et al. 2001). We found that NREM δ power density was significantly reduced when the Cre-GFP expressing virus was injected into Lkb1fl/fl mice (Fig. 6f). Analysis over 24 h indicated that significant reduction was observed over most of the daily cycle (Fig. 6i).
Discussion
Our results indicate that LKB1 is required for sleep regulation: it plays an important and conserved role by promoting sleep in both flies and mice. LKB1 plays this role in neurons in both species because gene targeting of Lkb1 in neurons led to reduction of sleep. In mice, with the additional advantage of EEG analysis, we find that LKB1 regulates sleep need as indicated by reduced NREM δ power density in Lkb1 knockdown mutant mice.
Sleep is important for animals. Sleep regulation is accomplished through 2 processes: circadian and sleep homeostatic (Borbely 1982; Borbely et al. 2016). The circadian clock regulates the timing of sleep, and homeostatic process regulates the sleep drive. Molecular mechanisms of the circadian clock have been revealed through research in Drosophila and other organisms (Hendricks et al. 2000; Shaw et al. 2000; Nitabach and Taghert 2008; Allada and Chung 2010; Mohawk et al. 2012). Although many sleep-related genes have been identified in sleep regulation (Cirelli 2009; Allada et al. 2017; Jan et al. 2020), our understanding of the mechanisms underlying sleep homeostatic regulation remains limited (Allada et al. 2017; Donlea et al. 2017).
Multiple regions in Drosophila and mouse brains have been implicated in sleep regulation. In flies, sleep is regulated by several regions including: the ILNv and DN1 clock neurons which are important for circadian control of sleep (Parisky et al. 2008; Shang et al. 2008; Sheeba et al. 2008; Chung et al. 2009; Shang et al. 2013; Kunst et al. 2014). And the mushroom bodies, the dorsal of fan-shaped body, the ellipsoid body, the pars intercerebralis, and glia (Joiner et al. 2006; Foltenyi et al. 2007; Crocker et al. 2010; Donlea et al. 2011; Guo et al. 2011; Seugnet et al. 2011; Liu et al. 2012; Ueno et al. 2012; Yi et al. 2013; Donlea et al. 2014; Park et al. 2014; Chen et al. 2015; Liu et al. 2016; Pimentel et al. 2016). In mammals, sleep is regulated by monoaminergic, cholinergic, glutamatergic, and GABAergic neurons that are distributed in multiple regions including the brain stem, the preoptic hypothalamus, the lateral hypothalamus, and the basal forebrain (Weber and Dan 2016; Saper and Fuller 2017; Scammell et al. 2017). It will be interesting to investigate whether Lkb1 functions in all or a limited subset of neurons to regulate sleep.
Lkb1 as a master kinase can regulate the activities of ARKs by phosphorylating the site in the active T loop equivalent to AMPK-T172 (Lizcano et al. 2004). Because both AMPK and SIK3 are involved in sleep regulation, it will be interesting to investigate downstream kinases mediating the function of Lkb1 in sleep regulation. Is it SIK3, AMPK, or other members of the ARKs? Our findings of allele-specific genetic interactions between Lkb1 and Ampk suggest that they could be upstream and downstream of each other in regulating sleep. Because of the lethality of double mutation combination of Sik3 and lkb1, we cannot rule out that Sik3 may also be downstream of Lkb1 in regulating Drosophila sleep. The Ca2+/calmodulin-dependent protein kinase kinase-2 (CaMKK2, also known as CaMKKβ) could phosphorylate AMPKα-T172 (Hawley et al. 2005; Hurley et al. 2005; Woods et al. 2005; Anderson et al. 2008), but CaMKK2 could not phosphorylate the equivalent sites in the other ARKs, including SIK3 (Fogarty et al. 2010). It will be interesting to investigate whether and how CaMKK2 regulates sleep.
In Drosophila, LKB1 functions through SIK3 which phosphorylates histone deacetylase 4 (HDAC4) to regulate lipid storage (Choi et al. 2015). It will be interesting to investigate whether HDAC4 is downstream of LKB1 in sleep regulation.
More importantly, an important question for further studies is whether Lkb1 regulation of sleep is related to its regulation of metabolism. Changes in energy homeostasis directly and reversibly influence the sleep/wake cycle (Collet et al. 2016). Some molecules involved in metabolism regulate sleep (Taheri et al. 2004; Bjorness and Greene 2009; Thimgan et al. 2010; Gerstner et al. 2011; Nixon et al. 2015; Grubbs et al. 2020). In Drosophila, starvation suppresses sleep without building up sleep drive (Thimgan et al. 2010). Lkb1 and its downstream components are involved in regulating metabolism, with examples such as LKB1-AMPK signaling in the liver regulating glucose homeostasis (Shaw et al. 2005), SIK3-HDAC4 regulating energy balance in Drosophila (Wang et al. 2011). Either LKB1 has 2 independent roles in sleep and metabolism or that its 2 roles are related.
Our recent in vitro biochemical discoveries of STE20 phosphorylation of AMPK and SIK3 (and other ARKs) raise more questions about physiological significance of any STE20 or any other ARK in sleep (Liu, Wang, Cui, Gao, et al. 2022; Liu, Wang, Cui, et al. 2022).
Data availability
Strains and plasmids are available upon request. All data necessary for confirming the conclusions of the article are present within the article and its supplementary data.
Supplemental material is available at GENETICS online.
Acknowledgments
We are grateful to Dr Bowen Deng for Ampk flies, to Ping-ping Yan, Lan Wang, and Yong-hui Zhang for technical assistance, to Drs Wei Yang and En-xin Zhou for Drosophila video tracing programs, to Dan Wang for mouse rearing, to members of the Rao lab for discussion, to the Bloomington Drosophila Stock Center for flies, to the Jackson Laboratory for mice, to CIBR, Peking-Tsinghua Center for Life Sciences, IDG/McGovern Institute for Brain Research at Peking University and Changping Laboratory for support.
Conflicts of interest
The authors declare no conflict of interests.