-
PDF
- Split View
-
Views
-
Cite
Cite
Deepshikha Dogra, Warakorn Kulalert, Frank C Schroeder, Dennis H Kim, Neuronal KGB-1 JNK MAPK signaling regulates the dauer developmental decision in response to environmental stress in Caenorhabditis elegans, Genetics, Volume 220, Issue 1, January 2022, iyab186, https://doi.org/10.1093/genetics/iyab186
Close - Share Icon Share
Abstract
In response to stressful growth conditions of high population density, food scarcity, and elevated temperature, young larvae of nematode Caenorhabditis elegans can enter a developmentally arrested stage called dauer that is characterized by dramatic anatomic and metabolic remodeling. Genetic analysis of dauer formation of C. elegans has served as an experimental paradigm for the identification and characterization of conserved neuroendocrine signaling pathways. Here, we report the identification and characterization of a conserved c-Jun N-terminal Kinase-like mitogen-activated protein kinase (MAPK) pathway that is required for dauer formation in response to environmental stressors. We observed that loss-of-function mutations in the MLK-1-MEK-1-KGB-1 MAPK pathway suppress dauer entry. A loss-of-function mutation in the VHP-1 MAPK phosphatase, a negative regulator of KGB-1 signaling, results in constitutive dauer formation, which is dependent on the presence of dauer pheromone but independent of diminished food levels or elevated temperatures. Our data suggest that the KGB-1 pathway acts in the sensory neurons, in parallel to established insulin and TGF- signaling pathways, to transduce the dauer-inducing environmental cues of diminished food levels and elevated temperature.
Introduction
Caenorhabditis elegans can enter an alternative developmental diapause state, known as dauer, in response to unfavorable environmental conditions—specifically increased population density, scarcity of bacterial food, and elevated temperature (Golden and Riddle 1982, 1984a, 1984b, 1984c). The dauer state is characterized by increased stress resistance and dramatic remodeling of anatomy and metabolism (Cassada and Russell 1975). The genetic analysis of dauer formation has served as an experimental paradigm for understanding how neuroendocrine signaling through conserved components such as the insulin, TGF-, and nuclear hormone receptor pathways control organismal physiology and modulate developmental plasticity (Riddle and Albert 1997; Hu 2007; Fielenbach and Antebi 2008; Baugh and Hu 2020).
Recent studies have defined the molecular basis for how C. elegans responds to increased population density through pheromone signaling. Dauer pheromone has been characterized as a mixture of ascarosides, several structurally related derivatives of the dideoxysugar ascarylose (Butcher et al. 2007), which act on diverse families of G-protein-coupled membrane receptors (GPCRs). Receptors encoded by srbc-64 and srbc-66 are involved in the perception of ascarosides ascr#1, ascr#2, and ascr#3, with probable role of additional GPCRs as the double mutant srbc-64; srbc-66 still forms dauer at high concentrations of these ascarosides (Kim et al. 2009). McGrath et al. (2011) identified two predicted GPCRs, srg-36 and srg-37, as ascr#5-specific receptors. Two additional receptors, DAF-37 and DAF-38, respond to ascr#2, ascr#3, and ascr#5 (Park et al. 2012). Pheromone signaling also modulates expression of DAF-7 (TGF- ligand) and insulin ligands (Ren et al. 1996; Schackwitz et al. 1996; Li et al. 2003), linking the activity of pheromone to canonical signaling pathways involved in the control of the dauer developmental decision.
In contrast, the mechanisms by which diminished levels of high-quality bacterial food or temperature can modulate the dauer decision remain less well understood. Food signal was first described as a compound present in bacterial extract that can inhibit dauer formation and initiate dauer recovery (Golden and Riddle 1982, 1984a). Bacterial fatty acids have been implicated in promoting dauer recovery (Kaul et al. 2014). Of note, recent studies have shown a role of Rictor/TORC2 and CaMKI in regulating the expression of TGF- ligand and insulin-like proteins in response to food signals in dauer entry (Neal et al. 2015; O’Donnell et al. 2018).
We previously described a genetic strategy to identify novel genes involved in dauer formation by undertaking a screen for suppressors of the daf-28(sa191) mutation (Kulalert and Kim 2013; Kulalert et al. 2017), which confers constitutive entry into dauer diapause (Malone et al. 1996; Li et al. 2003). Here, we have identified and characterized a c-Jun N-terminal Kinase (JNK)-like mitogen-activated protein kinase (MAPK) KGB-1 pathway that functions in the sensory neurons to promote dauer formation. We characterize the effect of loss of the KGB-1 pathway, as well as its hyperactivation in a VHP-1 MAPK phosphatase mutant, on the regulation of the dauer developmental decision. Our data suggest a role for KGB-1 signaling in transducing diminished bacterial food and elevated temperature cues, which are integrated with pheromone signaling to promote entry into dauer diapause.
Materials and methods
Strains
All C. elegans strains used in this study were maintained as previously described (Brenner 1974). The temperature sensitive strains were maintained at 16°C and all other strains were incubated at 20°C. The following is a complete list of all the strains used in this study:
| Strain . | Genotype . |
|---|---|
| N2 | Wild-type |
| KU21 | kgb-1(km21) |
| KB3 | kgb-1(um3) |
| ZD2520 | mek-1(qd375) |
| FK171 | mek-1(ks54) |
| ZD1645 | mlk-1(qd318) |
| ZD108 | mlk-1(km19) |
| FXO1729 | shc-1(tm1729) |
| VC822 | kgb-2(gk361) |
| VC8 | jnk-1(gk7) |
| BS3383 | pmk-3(ok169) |
| JT366 | vhp-1(sa366) |
| ZD2350 | vhp-1(sa366);kgb-1(km21) |
| ZD2638 | vhp-1(sa366);mek-1(qd375) |
| ZD110 | vhp-1(km20)/mIn1 |
| ZD2113 | mek-1(qd375);qdEx197[mek-1(+)] |
| ZD2110 | mek-1(qd375);qdEx198[mek-1(+)] |
| ZD2324 | mek-1(qd375);qdEx199[mek-1(+)] |
| ZD2351 | mek-(qd375);qdEx187[rab-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2508 | mek-(qd375);qdEx191[rab-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2325 | mek-1(qd375);qdEx200[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2326 | mek-1(qd375);qdEx201[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2543 | mek-1(qd375);qdEx202[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2506 | mek-1(qd375);qdEx189[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2507 | mek-1(qd375);qdEx190[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2513 | mek-1(qd375);qdEx192[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2569 | mek-1(qd375);qdEx194[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2570 | mek-1(qd375);qdEx195[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2571 | mek-1(qd375);qdEx196[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| CF1038 | daf-16(mu86) |
| ZD2518 | daf-16(mu86);vhp-1(sa366) |
| DR20 | daf-12(m20) |
| ZD2567 | daf-12(m20);vhp-1(sa366) |
| CB1376 | daf-3(e1376) |
| ZD2639 | daf-3(e1376);vhp-1(sa366) |
| TY3856 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))] |
| TY3862 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))]; daf-7(e1372) |
| ZD2640 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))]; vhp-1(sa366) |
| Strain . | Genotype . |
|---|---|
| N2 | Wild-type |
| KU21 | kgb-1(km21) |
| KB3 | kgb-1(um3) |
| ZD2520 | mek-1(qd375) |
| FK171 | mek-1(ks54) |
| ZD1645 | mlk-1(qd318) |
| ZD108 | mlk-1(km19) |
| FXO1729 | shc-1(tm1729) |
| VC822 | kgb-2(gk361) |
| VC8 | jnk-1(gk7) |
| BS3383 | pmk-3(ok169) |
| JT366 | vhp-1(sa366) |
| ZD2350 | vhp-1(sa366);kgb-1(km21) |
| ZD2638 | vhp-1(sa366);mek-1(qd375) |
| ZD110 | vhp-1(km20)/mIn1 |
| ZD2113 | mek-1(qd375);qdEx197[mek-1(+)] |
| ZD2110 | mek-1(qd375);qdEx198[mek-1(+)] |
| ZD2324 | mek-1(qd375);qdEx199[mek-1(+)] |
| ZD2351 | mek-(qd375);qdEx187[rab-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2508 | mek-(qd375);qdEx191[rab-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2325 | mek-1(qd375);qdEx200[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2326 | mek-1(qd375);qdEx201[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2543 | mek-1(qd375);qdEx202[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2506 | mek-1(qd375);qdEx189[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2507 | mek-1(qd375);qdEx190[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2513 | mek-1(qd375);qdEx192[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2569 | mek-1(qd375);qdEx194[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2570 | mek-1(qd375);qdEx195[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2571 | mek-1(qd375);qdEx196[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| CF1038 | daf-16(mu86) |
| ZD2518 | daf-16(mu86);vhp-1(sa366) |
| DR20 | daf-12(m20) |
| ZD2567 | daf-12(m20);vhp-1(sa366) |
| CB1376 | daf-3(e1376) |
| ZD2639 | daf-3(e1376);vhp-1(sa366) |
| TY3856 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))] |
| TY3862 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))]; daf-7(e1372) |
| ZD2640 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))]; vhp-1(sa366) |
| Strain . | Genotype . |
|---|---|
| N2 | Wild-type |
| KU21 | kgb-1(km21) |
| KB3 | kgb-1(um3) |
| ZD2520 | mek-1(qd375) |
| FK171 | mek-1(ks54) |
| ZD1645 | mlk-1(qd318) |
| ZD108 | mlk-1(km19) |
| FXO1729 | shc-1(tm1729) |
| VC822 | kgb-2(gk361) |
| VC8 | jnk-1(gk7) |
| BS3383 | pmk-3(ok169) |
| JT366 | vhp-1(sa366) |
| ZD2350 | vhp-1(sa366);kgb-1(km21) |
| ZD2638 | vhp-1(sa366);mek-1(qd375) |
| ZD110 | vhp-1(km20)/mIn1 |
| ZD2113 | mek-1(qd375);qdEx197[mek-1(+)] |
| ZD2110 | mek-1(qd375);qdEx198[mek-1(+)] |
| ZD2324 | mek-1(qd375);qdEx199[mek-1(+)] |
| ZD2351 | mek-(qd375);qdEx187[rab-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2508 | mek-(qd375);qdEx191[rab-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2325 | mek-1(qd375);qdEx200[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2326 | mek-1(qd375);qdEx201[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2543 | mek-1(qd375);qdEx202[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2506 | mek-1(qd375);qdEx189[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2507 | mek-1(qd375);qdEx190[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2513 | mek-1(qd375);qdEx192[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2569 | mek-1(qd375);qdEx194[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2570 | mek-1(qd375);qdEx195[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2571 | mek-1(qd375);qdEx196[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| CF1038 | daf-16(mu86) |
| ZD2518 | daf-16(mu86);vhp-1(sa366) |
| DR20 | daf-12(m20) |
| ZD2567 | daf-12(m20);vhp-1(sa366) |
| CB1376 | daf-3(e1376) |
| ZD2639 | daf-3(e1376);vhp-1(sa366) |
| TY3856 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))] |
| TY3862 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))]; daf-7(e1372) |
| ZD2640 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))]; vhp-1(sa366) |
| Strain . | Genotype . |
|---|---|
| N2 | Wild-type |
| KU21 | kgb-1(km21) |
| KB3 | kgb-1(um3) |
| ZD2520 | mek-1(qd375) |
| FK171 | mek-1(ks54) |
| ZD1645 | mlk-1(qd318) |
| ZD108 | mlk-1(km19) |
| FXO1729 | shc-1(tm1729) |
| VC822 | kgb-2(gk361) |
| VC8 | jnk-1(gk7) |
| BS3383 | pmk-3(ok169) |
| JT366 | vhp-1(sa366) |
| ZD2350 | vhp-1(sa366);kgb-1(km21) |
| ZD2638 | vhp-1(sa366);mek-1(qd375) |
| ZD110 | vhp-1(km20)/mIn1 |
| ZD2113 | mek-1(qd375);qdEx197[mek-1(+)] |
| ZD2110 | mek-1(qd375);qdEx198[mek-1(+)] |
| ZD2324 | mek-1(qd375);qdEx199[mek-1(+)] |
| ZD2351 | mek-(qd375);qdEx187[rab-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2508 | mek-(qd375);qdEx191[rab-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2325 | mek-1(qd375);qdEx200[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2326 | mek-1(qd375);qdEx201[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2543 | mek-1(qd375);qdEx202[elt-2p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2506 | mek-1(qd375);qdEx189[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2507 | mek-1(qd375);qdEx190[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2513 | mek-1(qd375);qdEx192[myo-3p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2569 | mek-1(qd375);qdEx194[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2570 | mek-1(qd375);qdEx195[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| ZD2571 | mek-1(qd375);qdEx196[bbs-1p::mek-1::F2A::mCherry::unc-54 3’UTR] |
| CF1038 | daf-16(mu86) |
| ZD2518 | daf-16(mu86);vhp-1(sa366) |
| DR20 | daf-12(m20) |
| ZD2567 | daf-12(m20);vhp-1(sa366) |
| CB1376 | daf-3(e1376) |
| ZD2639 | daf-3(e1376);vhp-1(sa366) |
| TY3856 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))] |
| TY3862 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))]; daf-7(e1372) |
| ZD2640 | cuIs5[myo2C::GFP+pRF4(rol-6(su1006))]; vhp-1(sa366) |
Dauer formation assays
The dauer assays, unless otherwise mentioned, were performed broadly as described (Neal et al. 2013), using the following-indicated pheromone mix, food sources, and temperatures. One hundred microliters of pheromone mix (containing ascarosides ascr#2, ascr#3, ascr#5, and ascr#8 each at a concentration of 20 µM in 10% ethanol) was added to 3.5 cm plates (volume ∼3 ml) made with Noble agar and without peptone, resulting in an effective plate concentration of 0.67 µM for each ascaroside. The plates were seeded with 20 µl of either concentrated heat-killed (60 mg/ml) or live (70 mg/ml) Escherichia coli strain OP50. Three to five gravid animals per individual plate were allowed to lay eggs for 3 h at assay temperature (25°C). The numbers of dauer and non-dauer animals were scored 72 h (heat-killed food) or 64 h (live food) post egg-lay midpoint. The assays with incubation at lower temperatures only differed in their length of incubation, the numbers of dauer and non-dauer animals were scored 96 h (20°C) and 120 h (16°C) post egg-lay midpoint.
Partial dauer characterization
Partial dauers are animals that possess some but not all characteristics of dauer. These dauer-like animals have a refractile intestine resembling a dauer and radial constriction of the body but not of the pharynx. Pumping is not completely halted, and fat stores are not accumulated but the cuticle structure resembles dauer alae (Vowels and Thomas 1992; Larsen et al. 1995; Ogg et al. 1997). We identified partial dauers morphologically and we used treatment with 1% SDS for definitive characterization. Previous studies have reported that partial dauers can survive SDS treatment longer than non-dauer animals but not as long as dauers (Nika et al. 2016). While non-dauer larvae and adult C. elegans animals are only able to survive in SDS for less than 10 min, dauers can easily survive longer than 60 min. Partial dauers on the other hand are able to survive for approximately 18–20 min, making their survival time much higher than the non-dauer animals but less than dauers.
Cloning and generation of transgenic lines
For the genomic rescue construct, the endogenous promoter of mek-1 (4.2 kb upstream), along with the entire gene and 3′ UTR (1 kb downstream) sequence were amplified from genomic DNA using forward primer 5′-GGCATAAAACATGCTGAAAAATAAATTC-3′ and reverse primer 5′-CCACGCCTAGACTGGTTTATC-3′. This PCR product was cloned into pUC19 backbone using NEBuilder HiFi DNA Assembly (New England Biolabs, Ipswich, MA, USA). The assembled plasmid was microinjected at 25 ng/µl concentration, along with ofm-1p::gfp as a co-injection marker at 50 ng/µl for endogenous mek-1 rescue in the mek-1 loss-of-function mutant background.
For tissue-specific expression constructs, MEK-1 cDNA (K08A8.1a.1) was amplified from N2 cDNA library using PCR. N2 cDNA library was created in lab using RNA isolation and reverse transcription. The cDNA expression was driven by various tissue-specific promoters viz. elt-2p (intestine-specific expression), myo-3p (body wall muscle-specific expression), rab-3p (pan-neuronal expression) and bbs-1p (pan-ciliated neuron-specific expression). The promoters along with the cDNA, F2A self-cleaving peptide, and mCherry sequence were all cloned into pPD95.75 plasmid backbone using NEBuilder HiFi DNA Assembly. The mCherry sequence was used to help confirm the expression of MEK-1 in the specific tissue, and the F2A self-cleaving peptide sequence ensured that MEK-1 and mCherry do not form a fusion protein.
The assembled plasmid for body-wall muscle-specific expression was microinjected at 25 ng/µl concentration, along with ofm-1p::gfp as a co-injection marker at 50 ng/µl for tissue-specific rescue of mek-1 in the mek-1 loss-of-function mutant background. The remaining assembled plasmids were microinjected at 5 ng/µl concentration (due to toxicity issues at higher concentration), along with ofm-1p::gfp as a co-injection marker at 100 ng/µl for tissue-specific rescue of mek-1 in the mek-1 loss-of-function mutant background.
Imaging
For measurement of cuIs5 GFP in the pharynx, animals were egg laid in the pheromone assay as described above and 24 h post egg-lay midpoint, the animals were mounted with 1 mM sodium azide onto slides with a 2% agarose pad. Slides were viewed using Zeiss AxioImager Z1 fluorescence microscope primarily with 20× objective. Fluorescence signals were recorded with a charge-coupled device camera (AxioCam) using constant exposure time without saturation. For quantification, maximum intensity values of GFP within the pharynx were calculated using FIJI software (Schindelin et al. 2012; Fiji, RRID: SCR_002285).
Statistical analysis
All statistical analysis was performed using the GraphPad Prism software (Graphpad Prism, RRID: SCR_002798). Statistical tests used are indicated in each figure legend, the asterisks on the figure indicate significant difference compared to the left-most bar.
Results
We previously reported the results of a genetic screen for suppressors of the dauer-constitutive (Daf-c) phenotype of the daf-28(sa191) mutant (Kulalert et al. 2017). Incomplete suppression of the Daf-c phenotype of the daf-28(sa191) mutant by daf-3 and daf-16 mutations (Malone et al. 1996; Li et al. 2003; Kulalert and Kim 2013) suggested that the identification of genetic suppressors of daf-28(sa191) might uncover additional novel pathways functioning in parallel to established signaling pathways activating the dauer developmental decision. Our prior characterization of the molecular mechanisms promoting dauer entry of the daf-28(sa191) mutant determined that activation of the Unfolded Protein Response and PEK-1 phosphorylation of eIF2 in the ASI neuron pair conferred the Daf-c phenotype (Kulalert and Kim 2013; Kulalert et al. 2017). We reported the isolation of multiple suppressor mutations in genes acting in this pathway downstream of UPR activation in the ASI neuron pair (Kulalert et al. 2017). We reasoned that we might also isolate suppressor mutations in novel pathways acting in parallel to this and previously characterized pathways leading to dauer formation. We recovered a strain carrying the qd318 mutation from a genetic screen for suppressors of the Daf-c phenotype of the daf-28(sa191) mutant (Kulalert et al. 2017). Molecular characterization (described in Kulalert et al. 2017) of this strain revealed a P365S substitution mutation in the mlk-1 gene encoding an MAPK kinase kinase. We sought to determine whether MLK-1 is also required for dauer entry in wild-type animals in the absence of the daf-28(sa191) mutation.
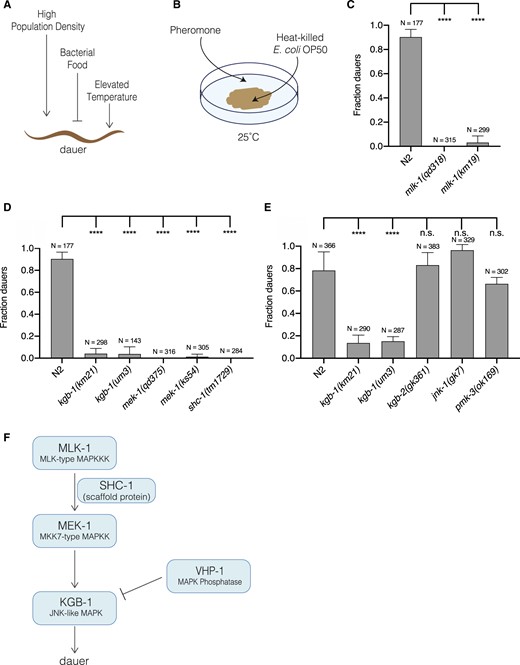
Induction of dauer formation under experimental conditions involves the addition of dauer pheromone, which is a combination of ascaroside molecules produced by C. elegans (Jeong et al. 2005; Butcher et al. 2007; Butcher et al. 2008; Pungaliya et al. 2009; Gallo and Riddle 2009), reduction of high-quality bacterial food E. coli OP50, and incubation at higher temperatures (Golden and Riddle 1984a, 1984b; Ailion and Thomas 2000; Neal et al. 2013). Figure 1A depicts an overview of the environmental conditions that affect the decision to enter dauer. We experimentally imposed environmental stressors of crowding, low food availability, and elevated ambient temperature in the laboratory, by exposing larvae to a combination of pheromone, heat-killed E. coli OP50, and incubation temperature of 25°C (Figure 1B). Under these conditions, we observed that >80% of wild-type larvae formed dauers (Figure 1C). In contrast, we observed that dauer formation was largely blocked in the mlk-1(qd318) mutant and a mutant carrying a deletion in the mlk-1 gene, mlk-1(km19).
KGB-1 pathway activation is required for dauer entry caused by environmental stress. (A) Schematic depiction of environmental conditions that promote and suppress dauer formation. High population density and elevated temperature promote (shown by pointing arrows), and abundant food supply suppresses (shown by stopped line) dauer formation. (B) Schematic of dauer inducing conditions: a combination of pheromone, heat-killed E. coli OP50, and 25°C, applied to noble agar plates to emulate the environmental conditions promoting dauer formation depicted in (A). (C) Fraction of wild-type (N2) animals, and mlk-1(qd318) and mlk-1(km19) mutant animals entering dauer in the presence of dauer inducing conditions. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD; N, total number of animals tested. (D) Fraction of wild-type (N2) animals, and kgb-1(km21), kgb-1(um3), mek-1(qd375), mek-1(ks54), and shc-1(tm1729) mutant animals entering dauer in the presence of dauer-inducing conditions. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD, N, = total number of animals tested. (E) Fraction of wild-type (N2) animals, and kgb-1(km21), kgb-1(um3), kgb-2(gk361), jnk-1(gk7) and pmk-3(ok169) mutant animals entering dauer in the presence of dauer inducing conditions. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD; N, total number of animals tested; n.s., not significant. (F) Schematic of KGB-1 pathway, a JNK-like MAPK pathway, composed of MLK-1 (MLK-type MAP3K), MEK-1 (MKK7-type MAP2K), SHC-1 (scaffold protein), KGB-1 (JNK-like MAPK) and VHP-1 (MAPK phosphatase).
MLK-1 has been previously characterized as acting upstream of the KGB-1 MAPK, which is homologous to the conserved JNK MAPK. MLK-1 phosphorylates the MAPK kinase MEK-1, a homolog of the MKK7 MAPKK, in the presence of the scaffold protein SHC-1, and MEK-1 phosphorylates and activates KGB-1 (Mizuno et al. 2004, 2008; Sakaguchi et al. 2004). All the kinase proteins in this pathway as well as the scaffold protein are essential for activation of KGB-1. The animals with loss-of-function mutations in genes encoding components of the KGB-1 pathway continued their reproductive growth, failing to enter dauer under these stressful conditions (Figure 1D). We determined that the KGB-1 MAPK is the only JNK MAPK required for dauer entry, as only kgb-1 loss-of-function mutants (km21 and um3) suppressed dauer entry, whereas the animals carrying mutations in the other two JNK homologs, jnk-1(gk7) and kgb-2(gk361), were able to enter dauer similar to wild-type animals in the presence of the dauer inducing conditions (Figure 1E). The KGB-1 pathway was reported to have cross-talk with PMK-3 (p38 MAPK) pathway in axon regeneration where both the pathways are required (Nix et al. 2011), but we observed that the pmk-3 loss-of-function mutant exhibited wild-type dauer entry (Figure 1E). Our data establish that the MLK-1-(SHC-1)-MEK-1-KGB-1 JNK MAPK pathway is required for dauer entry (Figure 1F).
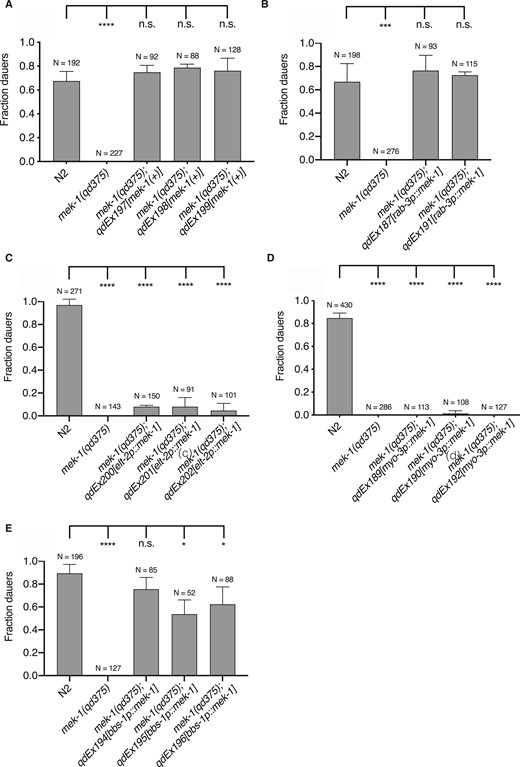
To determine the tissue and cell types in which the KGB-1 pathway functions to promote dauer entry, we conducted tissue-specific rescue experiments of the mek-1(qd375) mutant using transgenes expressing the mek-1 cDNA under the control of heterologous promoters. We fully rescued the Daf-d phenotype of the mek-1(qd375) mutant when expressing mek-1 under its endogenous promoter and 3′UTR in an extrachromosomal array (Figure 2A). We observed that expression of mek-1 in the nervous system of mek-1(qd375) mutant animals, under the control of the pan-neuronal promoter, rab-3p, resulted in rescue of the dauer formation phenotype, whereas expression of mek-1 in the intestine or body wall muscles failed to rescue the Daf-d phenotype of the mek-1(qd375) mutant (Figure 2, B–D). In particular, we introduced expression of mek-1 under the bbs-1p promoter, which directs expression in all ciliated neurons, and observed that expression in this specific subset of neurons rescued the Daf-d phenotype of mek-1(qd375) mutant animals (Figure 2E). Ciliated neurons encompass the chemosensory nervous system of C. elegans, and a number of specific sensory neurons have been implicated in roles in entry into, and exit from, dauer diapause (Albert et al. 1981; Bargmann and Horvitz 1991; Schackwitz et al. 1996; Ren et al. 1996; Li et al. 2003; Bargmann 2006; Ludewig and Schroeder 2013), but we were unable to further define specific neurons involved in dauer entry using neuronal cell type-specific rescue experiments. These data suggest either limitations of our experimental approach or the function of KGB-1 across multiple ciliated chemosensory neurons in dauer formation.
KGB-1 pathway acts in the chemosensory neurons to regulate dauer diapause. Fraction of wild-type (N2) animals, mek-1(qd375) mutant animals (non-sibling control) and mek-1(qd375) animals with extrachromosomal expression of (A) endogenous mek-1 gene (B) mek-1 gene under pan-neuronal promoter, rab-3p (C) mek-1 gene under intestine-specific promoter, elt-2p (D) mek-1 gene under body-wall muscle-specific promoter, myo-3p (E) mek-1 gene under pan-ciliated neuron-specific promoter, bbs-1p, entering dauer in the presence of pheromone and heat-killed E. coli OP50 at 25°C. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD; N, total number of animals tested; n.s., not significant.
Having observed the effect of loss of KGB-1 signaling on blocking entry into dauer diapause, we sought to examine if increased KGB-1 signaling would confer the opposite phenotype of enhancing dauer formation. The VHP-1 MAPK phosphatase was previously characterized as a negative regulator of the KGB-1 MAPK, and diminished activity of VHP-1 resulted in hyperactivation of KGB-1 (Mizuno et al. 2004). A strain carrying a vhp-1(km20) deletion was previously noted to exhibit early larval lethality (Mizuno et al. 2004), but a viable vhp-1 mutant carrying a nonsense mutation, vhp-1(sa366) has also been reported (Choy and Thomas 1999), and thus we examined this mutant for a dauer formation phenotype.
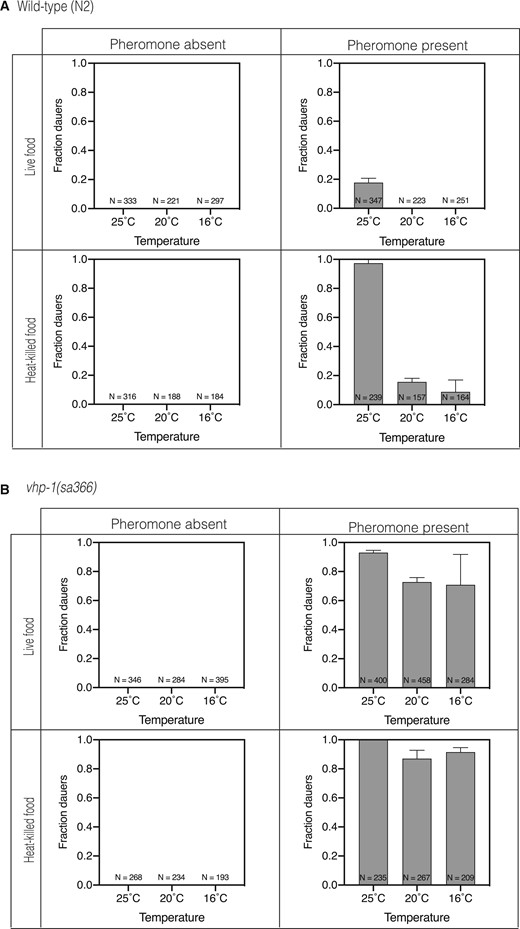
We examined wild-type and mutant larvae under 12 different sets of conditions derived from combinations of the presence or absence of pheromone, the presence of high-quality live bacterial food or poor quality heat-killed bacterial food, and three temperatures (25°C, 20°C, 16°C). The experimental data are presented in a 2 × 2 array, with each cell distinguished by the presence or absence of pheromone and whether larvae were exposed to live bacterial food or heat-killed bacterial food. Within each cell, the results of the three different incubation temperatures are presented. As we indicated above, optimal induction of dauer formation in wild-type animals was observed in the presence of dauer pheromone and heat-killed bacterial food at the elevated temperature of 25°C (Figure 3A). We did not observe dauer formation in the vhp-1(sa366) mutant in the absence of pheromone, even under conditions of bacterial food deprivation and elevated temperature, just as we observed for wild-type larvae. In the presence of pheromone and heat-killed food, we observed high-level dauer formation in vhp-1(sa366) larvae as was observed for wild-type animals. However, in contrast to what we observed for wild-type larvae, we detected equivalent high levels of dauer entry even at the lower temperatures of 20°C and 16°C under these conditions (Figure 3B). More dramatic differences were observed under conditions in the presence of pheromone and live bacterial food. In wild-type animals, the presence of live bacterial food largely suppressed dauer formation, but in vhp-1(sa366) animals, we observed marked dauer entry, equivalent to levels observed in the presence of heat-killed bacterial food.
Increased activation of the KGB-1 pathway in the vhp-1 mutant leads to constitutive dauer formation in the presence of pheromone. Fraction of (A) wild-type animals (B) vhp-1(sa366) mutant animals entering dauer under various combinations of dauer inducing conditions. The first row shows fraction dauers in the presence of live E. coli OP50 and the second row shows fraction dauers in the presence of heat-killed E. coli OP50. The first column shows fraction dauers in the absence of pheromone and the second column shows fraction dauers in the presence of pheromone. Each graph in the four panels shows fraction dauers at 25C, 20°C and 16°C, respectively. Plotted is mean + SD; N, total number of animals tested.
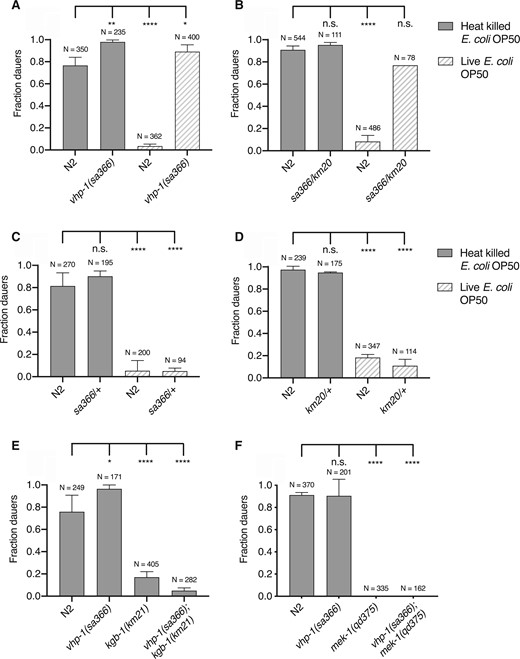
We confirmed that vhp-1(sa366) is a hypomorphic allele by genetic analysis of trans-heterozygote animals using wild-type animals and the vhp-1(km20) null mutant (Figure 4, A–D). We confirmed that the absence of kgb-1 suppresses dauer formation in the vhp-1(sa366) mutant animals. We also confirmed that dauer formation in the vhp-1(sa366) mutant was suppressed by mutation in the KGB-1 pathway, specifically a mutation in the mek-1 gene encoding the MAPKK MEK-1 that phosphorylates KGB-1 (Figure 4, E and F). In the absence of phosphorylation of KGB-1, reduction of function of the VHP-1 phosphatase would not be expected to affect KGB-1 activity.
Dauer formation of vhp-1(sa366) mutant animals is due to hyperactivation of KGB-1 pathway. (A–D) Fraction of wild-type (N2) animals and (A) vhp-1(sa366) homozygous mutant animals (B) vhp-1(sa366/km20) heterozygous mutant animals (C) vhp-1(sa366/+) heterozygous mutant animals (D) vhp-1(km20/+) heterozygous mutant animals, entering dauer in the presence of pheromone, 25°C and either heat-killed (solid bars) or live (patterned bars) E. coli OP50. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD, N = total number of animals tested, n.s. = not significant. (E, F) Fraction of wild-type (N2) animals, vhp-1(sa366), (E) kgb-1(km21) and vhp-1(sa366); kgb-1(km21), (F) mek-1(qd375) and vhp-1(sa366); mek-1(qd375) mutant animals, entering dauer in the presence of pheromone, 25°C and heat-killed E. coli OP50. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD; N, total number of animals tested; n.s., not significant.
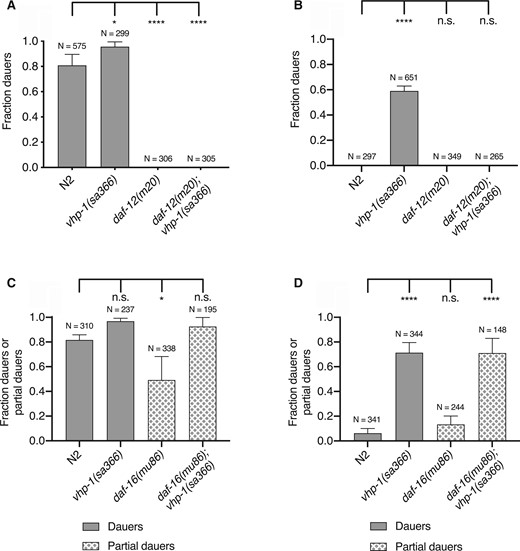
These data suggest that increased activity of the KGB-1 pathway in the vhp-1(sa366) mutant can substitute for the requirement for diminished bacterial food availability and elevated temperature in the induction of dauer formation, whereas the requirement for pheromone is unaffected by increased KGB-1 activity. We next sought to define the genetic interactions among KGB-1 signaling and established pathways involved in the dauer developmental decision. We determined that the enhanced dauer entry phenotype of vhp-1(sa366) mutant larvae was suppressed by daf-12 (Figure 5, A and B), as is the case with all known dauer-constitutive mutants (Vowels and Thomas 1992). We observed that the daf-16(mu86); vhp-1(sa366) mutant in the presence of pheromone resulted in the formation of partial dauers, regardless of whether live or heat-killed bacterial food was present (Figure 5, C and D). Partial dauer formation was also observed in double mutants involving daf-16 and dauer-constitutive mutants, including daf-28(sa191) (Vowels and Thomas 1992; Malone et al. 1996), but not observed in daf-2; daf-16 double mutants. The observation of partial dauers in the daf-16(mu86); vhp-1(sa366) mutant animals suggests that the KGB-1 signaling can activate the developmental decision to commit to dauer entry and partially proceed independent of DAF-16, but that the full execution of dauer program requires DAF-16 activity.
KGB-1 pathway functions upstream of the steroidal hormone pathway and in parallel to the insulin signaling pathway. (A, B) Fraction of wild-type (N2) animals and vhp-1(sa366), daf-12(m20) and daf-12(m20); vhp-1(sa366) mutant animals entering dauer in the presence of pheromone, 25°C and (A) heat-killed E. coli OP50 (B) live E. coli OP50. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD; N, total number of animals tested; n.s., not significant. (C, D) Fraction of wild-type (N2) animals and vhp-1(sa366), daf-16(mu86) and daf-16(mu86); vhp-1(sa366) mutant animals entering dauer in the presence of pheromone, 25°C and (C) heat-killed E. coli OP50 (D) live E. coli OP50. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD; N, total number of animals tested; n.s., not significant.
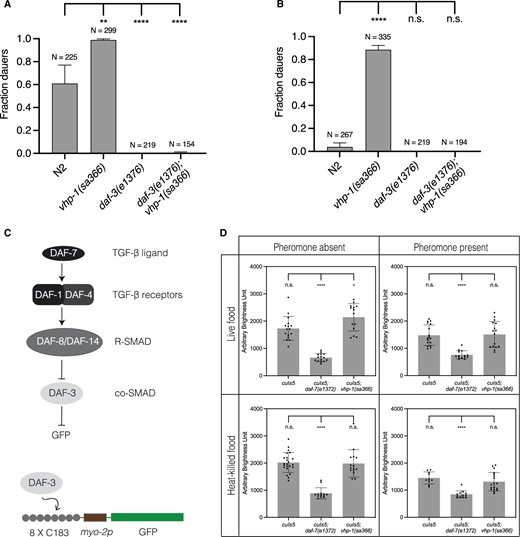
We observed that daf-3(e1376); vhp-1(sa366) mutants did not enter dauer in the presence of pheromone (Figure 6, A and B). One interpretation of this observation was that KGB-1 signaling might function upstream of DAF-7-DAF-3 signaling. Alternatively, because pheromone is required for vhp-1(sa366)-enhanced dauer formation, we considered the possibility that KGB-1 signaling might function in parallel with DAF-7-DAF-3 signaling, with the daf-3 mutation attenuating the effect of pheromone. Indeed, daf-7 mutants exhibit a dauer-constitutive phenotype in the absence of pheromone, and pheromone downregulates daf-7 expression in the ASI sensory neurons (Thomas et al. 1993; Ren et al. 1996; Schackwitz et al. 1996). To distinguish between these possibilities, we utilized a previously described reporter of DAF-3 activity, cuIs5 (Thatcher et al. 1999; Reiner et al. 2008). This reporter consists of myo-2 promoter, preceded by tandem copies of C183 portion of C-subelement, driving GFP expression in the pharynx. DAF-3 specifically recognizes and binds to C183 causing a suppression of GFP expression when it is not inhibited by DAF-7 (Figure 6C). As expected and has been reported, in the daf-7 mutant background, DAF-3 reporter activity is diminished. The presence of pheromone also decreased DAF-3 reporter activity relative to conditions in the absence of pheromone although no difference was observed in the daf-7 mutant, consistent with the daf-7 mutant mimicking conditions of pheromone presence. If vhp-1(sa366) promoted dauer entry by modulating DAF-3 activity, we would expect DAF-3 reporter activity to be similarly diminished in the vhp-1(sa366) background in the presence of pheromone. We observed that DAF-3 reporter activity was not affected by the vhp-1(sa366) mutation (Figure 6D). These data suggest that the vhp-1(sa366) mutation and corresponding KGB-1 activity function in parallel to TGF- signaling to promote dauer entry, and indicate that pheromone may act in part through modulation of the DAF-3-dependent signaling pathway.
KGB-1 pathway functions in parallel to TGF-β signaling pathway in regulation of dauer diapause. (A, B) Fraction of wild-type (N2) animals, vhp-1(sa366), daf-3(e1376) and daf-3(e1376); vhp-1(sa366) mutant animals entering dauer in the presence of pheromone, 25°C and (A) heat-killed E. coli OP50 (B) live E. coli OP50. Statistical analysis conducted with ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. Plotted is mean + SD, N = total number of animals tested, n.s. = not significant. (C) Overview of TGF-β signaling pathway and schematic of cuIs5—DAF-3 reporter transgene. (D) Quantification of GFP expression from DAF-3 reporter (cuIs5). Each graph in the four panels shows GFP expression in wild-type background, daf-7(e1372) and vhp-1(sa366) mutant backgrounds, respectively. The first row shows fraction dauers in the presence of live E. coli OP50 and the second row shows fraction dauers in the presence of heat-killed E. coli OP50. The first column shows fraction dauers in the absence of pheromone and the second column shows fraction dauers in the presence of pheromone. Plotted is mean + SD, each dot represents an animal; n.s., not significant.
Discussion
Dauer entry represents an integrated organismal response to environmental stress during development. Stress-activated MAPK signaling pathway have evolutionarily conserved roles in a wide range of stress responses in diverse species, and the KGB-1 JNK MAPK pathway has been implicated in the C. elegans response to multiple stressors, including heavy metals (Koga et al. 2000; Mizuno et al. 2004), the ER toxin tunicamycin (Mizuno et al. 2008), and food deprivation in the context of lifespan extension through intermittent fasting (Uno et al. 2013). KGB-1 is also required for resistance to pathogenic bacteria (Kim et al. 2004) and pore-forming toxins (Huffman et al. 2004). Here, our data suggest a role for KGB-1 signaling in the dauer developmental decision, with increased KGB-1 signaling in the vhp-1 mutant enabling dauer formation independent of bacterial food availability and temperature. Previous studies establishing an age-dependent role of KGB-1 pathway described interaction with insulin signaling pathway, specifically DAF-16 (Twumasi-Boateng et al. 2012; Liu et al. 2018). Our epistasis analysis suggests that the KGB-1 pathway functions in parallel to insulin signaling in the decision to enter dauer diapause, but that full execution of the dauer program requires DAF-16 as indicated by the formation of incomplete, or partial dauers in the absence of DAF-16.
Early studies established that the ratio of pheromone to bacterial food is more important than the absolute concentration per se in regulating dauer entry. Using serial dilutions of both pheromone and food concentrations, Riddle and colleagues showed that dauer phenotype is dependent on the relative concentration of the signals (Golden and Riddle 1982, 1984a). Higher pheromone concentration induces a greater fraction of the population to enter dauer but this effect saturates at a fraction dependent on the total amount of bacterial food present (Golden and Riddle 1984b). Similarly, increases in temperature enhance dauer entry in the presence of a constant pheromone to food ratio (Golden and Riddle 1984b, 1984c). Our results suggest a role of the KGB-1 pathway in the interpretation of the pheromone to food ratio in the dauer developmental decision. Whereas the prior identification of dauer-defective mutants and, correspondingly, signaling pathways that promote dauer formation have largely been in the experimental context of suppression of Daf-c mutations, recent studies have focused on the genetic characterization of dauer entry in wild-type larvae in response to environmental cues. Of note, mutants of Rictor/TORC2 exhibit high temperature (27°C) induced dauer entry, in a manner that was also not affected by bacterial food quality, and the Rictor/TORC2 pathway was implicated in relaying food-sensing information from the intestine to the nervous system (O’Donnell et al. 2018). Whereas rict-1 mutants modulated neuronal expression of daf-7, our data suggest that KGB-1 signaling mediates food sensitivity in parallel to the DAF-7/TGF- signaling pathway.
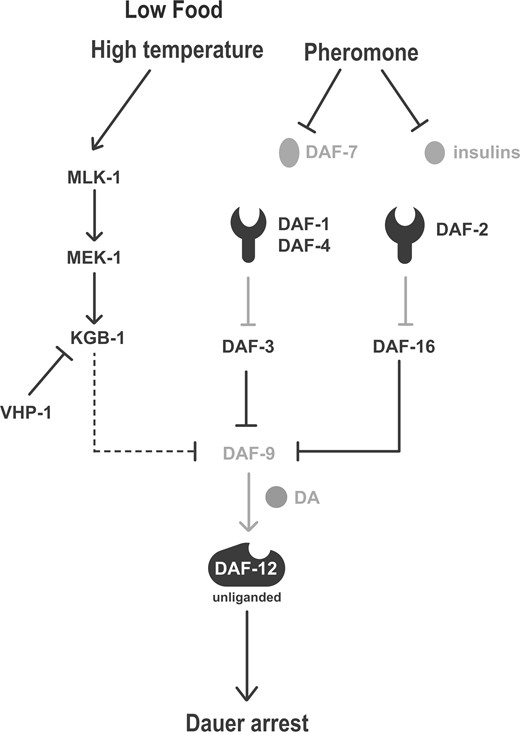
Our epistasis analysis suggests that KGB-1 signaling functions in parallel to the insulin/DAF-16 and DAF-7/TGF- signaling pathways are also consistent with the KGB-1 pathway functioning downstream of nutrient and/or temperature inputs into the dauer decision, independent of pheromone. Pheromone has been shown to modulate the expression of DAF-7 (Ren et al. 1996; Schackwitz et al. 1996) and insulin ligands of DAF-2 (Li et al. 2003; Cornils et al. 2011; Wong et al. 2020) consistent with a role for the insulin/DAF-16 and DAF-7/TGF- signaling pathways downstream of pheromone input, and unlike wild-type animals or vhp-1 mutant animals, dauer-constitutive mutants of the insulin/DAF-16 and DAF-7/TGF- signaling pathways can form dauer independent of the presence of pheromone. Our study suggests that the activity of a conserved stress-activated KGB-1 signaling in the sensory nervous system is required for the dauer developmental decision in response to diminished food availability as well as increased temperature, activating dauer entry in the presence of pheromone in parallel to established neuroendocrine pathways involved in the regulation of dauer formation (Figure 7).
Model figure depicting KGB-1 pathway functioning parallel to TGF-β and insulin signaling pathways in response to stress conditions of diminished food and elevated temperature to regulate dauer formation. Dashed line depicts tentative placement of KGB-1 pathway upstream of DAF-9. DA, dafachronic acids.
Data availability
Strains are available upon request. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Acknowledgments
The authors thank the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), for providing strains. They also thank members of the Kim lab for helpful conversations and feedback on the manuscript and figures.
Funding
This work is supported by NIGMS grants R01GM084477 and R35GM141794 to D.H.K. and R35GM131877 to F.C.S.
Conflict of interest
The authors declare that there is no conflict of interest.
Literature cited
Author notes
Present address: Metaorganism Immunity Section, Laboratory of Host Immunity and Microbiome, National Institute of Allergy and Infectious Diseases, National Institute of Health, Bethesda, MD 20892, USA.