-
PDF
- Split View
-
Views
-
Cite
Cite
Carlos Castillo‐Solórzano, Peter Carrasco, Gina Tambini, Susan Reef, Monica Brana, Ciro A. de Quadros, New Horizons in the Control of Rubella and Prevention of Congenital Rubella Syndrome in the Americas, The Journal of Infectious Diseases, Volume 187, Issue Supplement_1, May 2003, Pages S146–S152, https://doi.org/10.1086/368034
Close - Share Icon Share
Abstract
Data from the regional measles surveillance system have documented widespread rubella virus circulation in many different countries in the Americas. In response to the ongoing endemic incidence of the disease and the potential for a major rubella epidemics in the region, the Pan American Health Organization Technical Advisory Group on Vaccine Preventable Diseases recommended the implementation of a regional initiative to strengthen rubella and congenital rubella syndrome (CRS) preventive efforts in 1997. This article summarizes and highlights the progress toward accelerated rubella control and CRS prevention in the English‐speaking Caribbean and in Chile, Costa Rica, and Brazil. Useful knowledge is being generated for the adaptation of similar rubella strategies elsewhere. The findings also document the feasibility of implementing the recommended strategies and their rapid impact on disease burden.
Rubella, usually a mild febrile rash illness in children and adults, can produce devastating consequences when a woman becomes infected in early pregnancy. The sequels of infection during pregnancy include miscarriage, stillbirth, and a series of birth defects known as congenital rubella syndrome (CRS). Since 1969, rubella‐containing vaccines have been shown to be safe and effective in preventing rubella and CRS.
Data generated by a regional measles surveillance system developed by the Pan American Health Organization (PAHO) document widespread rubella virus circulation in many countries in the Americas. Some countries also have CRS cases. In the Western Hemisphere, an estimated 20,000 infants with CRS are born annually—even in the absence of major epidemics [1].
In response to the ongoing rubella virus circulation and the potential for major rubella epidemics in the region, the PAHO Technical Advisory Group on Vaccine Preventable Diseases recommended in 1997 that a regional initiative be implemented to strengthen rubella and CRS prevention efforts. To achieve this initiative, PAHO developed a rubella control and CRS strategy [2] that includes the introduction of rubella‐containing vaccines into routine childhood immunization programs, vaccination of women at childbearing age, development of specific vaccination strategies for accelerated rubella control and CRS prevention, support to countries in the development of integrated surveillance systems for measles and rubella, implementation of a CRS surveillance system, and rubella virus isolation. Here we summarize the progress made toward rubella control and CRS prevention with emphasis on experience gained in the accelerated control of rubella in the English‐speaking Caribbean and in Chile, Costa Rica, and Brazil.
Methods
Data from an integrated measles‐rubella surveillance system and a CRS surveillance system were analyzed to assess the burden of rubella and CRS disease. Coverage achieved and changes in the epidemiology of rubella and CRS were reviewed after the accelerated rubella vaccination campaign in the English‐speaking Caribbean and in Chile, Brazil, and Costa Rica when possible. Results of cost analysis and cost‐benefit studies were reviewed to assess the cost‐benefit of elimination of rubella in the Caribbean.
Integration of measles and rubella surveillance system. In 1994, as part of the documentation of measles eradication in the Americas, a regional measles eradication surveillance system was developed [3]. Since 1996, suspected measles cases that were IgM negative for measles have been tested for rubella IgM antibodies. In 1999, the system was expanded to a measles‐rubella integrated surveillance system to allow for simultaneous laboratory analysis. The objectives of the rubella component of the integrated surveillance system are to determine where the virus is circulating, to detect rubella cases in a timely manner for implementation of outbreak control measures, to quantify the magnitude of the problem, and to provide evidence of the effect of interventions [2].
For purposes of integrated surveillance, a patient suspected by a health worker of having measles or rubella infection is considered a “suspected” measles or rubella case. Typically these patients present with fever and a generalized maculopapular rash. Serology for rubella IgM antibodies and measles IgM antibodies should be obtained within 30 days after rash onset from all suspected measles and rubella cases. Rubella cases are classified as either laboratory or clinically confirmed. A confirmed case of rubella is defined as a person who has laboratory‐confirmed rubella (IgM+) or a person who meets the clinical case definition and is epidemiologically linked to a laboratory‐confirmed case. A clinically confirmed rubella case meets the clinical case definition but has had no serologic or virologic testing and is not epidemiologically linked to a laboratory‐confirmed case. A clinically confirmed case is considered a failure of the surveillance system. [4]. To manage the simultaneous surveillance of two diseases while maintaining the ability to analyze them separately, the concepts of initial and final diagnosis were introduced [5].
CRS surveillance. In 1998, a PAHO working group developed guidelines for CRS surveillance. The guidelines indicate that the main purpose of CRS surveillance is to identify infants <12 months old with suspected CRS. In order to identify these infants, all health care workers should suspect CRS in an infant whose mother had laboratory‐confirmed rubella infection during pregnancy and/or one or more of the following birth outcomes: congenital cataracts, hepatosplenomegaly, patent ductus arteriosus, purpura, or hearing impairment. In turn, the suspected cases of CRS should have serologic testing for rubella IgM antibodies and be classified by a standardized case definition as confirmed, clinically confirmed, clinical infection only, or discarded [5].
A confirmed case of CRS is defined as a child initially suspected of CRS with congenital anomalies compatible with CRS and laboratory evidence of rubella virus infection documented in the first year of life. At birth, the serum of an infant with CRS contains maternally derived rubella IgG antibodies as well as IgG and IgM antibodies synthesized by the fetus. In infants with CRS, rubella IgM can be detected in nearly 100% of the cases at age 0–5 months and in about 60% at age 6–12 months [6]. A clinically confirmed CRS case is an infant in whom a health care worker initially suspected CRS, but no laboratory confirmation of rubella infection was available—in general due to the absence of appropriate laboratory testing procedures. Since the presence or absence of rubella infection could not be determined, these cases are considered failures of the CRS surveillance system. A confirmed case of congenital rubella infection without CRS is an infant who has no clinical findings compatible with CRS but laboratory evidence of rubella virus infection. These cases should be classified only as congenital rubella infection, not as CRS.
Molecular typing. Since 1999, clinical specimens such as throat swabs have been collected from rubella and CRS patients [5]. Isolation of rubella virus from clinical specimens by culture in Vero cells, extraction of the single‐strand rubella virus RNA from the cultures, and amplification of the E1 gene (by reverse transcriptase polymerase chain reaction) were done as previously described [7]. Molecular typing is a laboratory method that is necessary to document the origin and epidemiology of the rubella virus strain.
Cost analysis. To estimate the cost‐benefit of eliminating rubella and CRS in the English‐speaking Caribbean, a review was undertaken of the results of an exercise done at the 1996 PAHO annual meeting of immunization managers of the English‐speaking Caribbean countries together with cost‐benefit studies conducted by several countries in that subregion [8].
Rubella vaccination strategies. Countries in the Americas are at different stages of rubella control and CRS prevention strategies. In general, the majority recommend the incorporation of a rubella‐containing vaccine in routine childhood programs at age 12 months and as part of the complementary follow‐up measles vaccination campaigns (a component of the PAHO measles eradication strategy) that seek to reach children aged 1–4 years at least every 4 years based on coverage obtained in routine services [9].
Countries ready to accelerate rubella control and/or CRS prevention should rapidly introduce a rubella‐containing vaccine into the adult population in addition to routine childhood vaccination [10, 11]. In order to accelerate CRS prevention, countries are advised to conduct a one‐time mass vaccination campaign that targets all females aged 5–39 years with measles and rubella‐containing vaccines. With this strategy, the number of CRS cases drops significantly, but as men remain susceptible, the virus continues to circulate. However, for countries that seek an accelerated rubella control strategy, a one‐time mass vaccination campaign (with a measles and rubella‐containing vaccine) is recommended for both males and females aged 5–39 years. This strategy will interrupt rubella virus transmission.
Results
Monitoring Rubella and CRS in the Americas
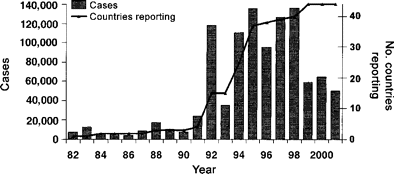
Rubella. Before 1992, only 4 of 44 countries and territories reporting to PAHO notified of rubella cases. By 1994, this number had increased to 25 countries and territories, and since 1998 all 35 countries and 9 territories report rubella cases. During 1998, there were 135,947 rubella cases reported (figure 1); however, 3 countries (Argentina, Mexico, and Venezuela) accounted for the majority (92%) of these cases. Since 1999, there has been a significant decrease in the number of reported rubella cases (58,764): Mexico, Venezuela, Brazil, and Argentina accounted for 89% of the cases. Most reported cases were clinically diagnosed and less than 20% had either a laboratory or epidemiologic link. In 2000 and 2001, there were 64,431 and 50,304 cases of rubella reported, respectively.
Rubella cases in the Americas, 1982–2001. Data for 44 countries and territories were compiled by the Pan American Health Organization.
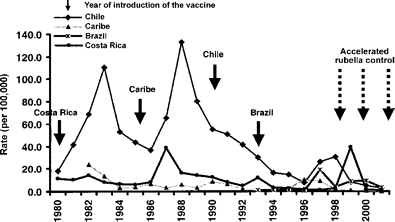
English‐speaking Caribbean. In 1991, the Caribbean launched the measles elimination campaign and countries started to incorporate rubella‐containing vaccine. By 1995, 11 of the 18 countries included this vaccine. From 1991 to 1994, the incidence of measles decreased from 6.49 cases per 100,000 population to less than 2.0 cases per 100,000 population. However, beginning in 1995, renewed rubella activity was observed with an incidence of 3.3 cases per 100,000 that rose to 10.3 per 100,000 persons by 1997 (figure 2). Significant rubella activity was first confirmed in Jamaica and Barbados in 1995, followed by Trinidad and Tobago in 1996 and Belize and Guyana in 1997. By age group, 53.0% of laboratory‐confirmed cases occurred in persons ⩾15 years old. Before the introduction of vaccine, most cases occurred in children <9 years old.
Rubella incidence in countries with accelerated rubella control initiatives, 1980–2001. Data from Pan American Health Organization.
After an adult rubella mass vaccination campaign in 1998 in the English‐speaking Caribbean, the incidence of rubella again decreased to 2 per 100,000 persons. Of the cases reported, 92% (124/135) occurred in countries where vaccination activities had not been initiated. Rubella cases continued to trend downward, with cases reported primarily in countries that needed to complete vaccination campaigns. From 1999 to 2001, the incidence decreased from 1.3 to 0.08 per 100,000 persons, respectively. In 2000, over 50% of the 19 cases were associated with importations and by 2001 Belize was the only country with confirmed rubella circulation.
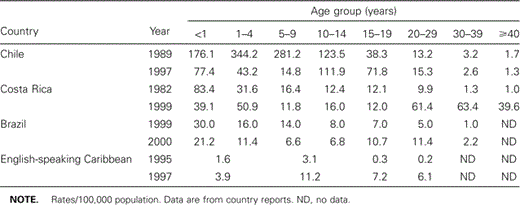
Chile. Chile established rubella surveillance in 1998 after two major rubella epidemics were documented in 1983 and 1988 (figure 2). In 1990 a routine childhood measles‐mumps‐rubella (MMR) program was introduced and in 1993 an MMR booster dose was added for 6‐year‐old children. This program was highly successful, and a steady decrease of rubella cases was observed from 41.4 cases per 100,000 to 7.6 per 100,000, respectively, between 1992 and 1996. Nevertheless, during 1997 and 1998, another rubella epidemic occurred, reaching an incidence of 30.2 cases per 100,000 population in 1998. Over 70% of the cases were among persons aged 10–29, unlike 1989 when over 70% of cases occurred among children ⩽10 years old (table 1) [12]. In 1999, a rubella vaccination campaign targeted women aged 10–29 and the reported national vaccination coverage reached was 98%. These efforts resulted in a significant decrease in the incidence of reported rubella cases to 2.8 per 100,000 population in 2001 (figure 2); the majority were in children <1 year old. However, only 1% of these cases were laboratory confirmed.
Shift in rubella incidence rates by age group in countries with accelerated rubella control initiatives.
Costa Rica. In 1972, rubella vaccine was introduced into the national childhood vaccination schedule and rubella surveillance was initiated. In 1986, the single antigen rubella‐containing vaccine was replaced with MMR. In 1992, an MMR booster dose was added at age 7 years, resulting in a decline in the number of rubella cases. During the last 14 years, three epidemic outbreaks of rubella occurred: 1987–1988 (55/100,000 population), 1993–1994 (15/100,000), and 1998–1999 (42/100,000) (figure 2). These outbreaks pointed to a shift in the age distribution of cases. The age group 15–24 years showed a progressive reduction in the proportion of cases (45% in 1987–1988, 25% in 1993–1994, and 11% in 1998–1999), while cases rose steadily in the 25‐ to 44‐year‐old group (23%, 31%, and 41%) in the same periods [13]. In the country’s last outbreak, the greatest risk was among persons aged 30–39 years (63.4/100,000 population), followed by persons aged 20–29 years (61.4/100,000) (table 1) [14]. After an adult rubella mass campaign, the last case of rubella was confirmed in August 2001.
Brazil. In 1992, Brazil began to introduce the MMR or measles‐rubella (MR) vaccine into the basic immunization schedule, and all 27 states included the vaccine by 2000. In 1992, there were 2286 (1.5/100,000) rubella cases reported. In 1997, the incidence of rubella increased to 20.6 per 100,000 but declined in 1999–2000 to 9.9 per 100,000 population (figure 2). Until 1999, the highest incidence of rubella was among children <15 years old. However, in 1999–2000, the highest incidence was in persons 15–29 years old [15] (table 1). This shift in transmission toward susceptible young adults is related to the gradual introduction of the MMR vaccine and the 95% vaccination coverage achieved in children aged 1–11 years in most Brazilian states between 1992 and 2000.
CRS
In the Americas, CRS is tracked by a passive surveillance system and there are limited surveillance data on the incidence of CRS. Most reports of CRS are from the English‐speaking Caribbean. In 1998, 32 countries reported 15 CRS cases; in 2000, 35 countries reported 84 cases. Due to the undernotification of this illness, this figure probably represents only the tip of the iceberg. In 1997–1999, the English‐speaking Caribbean reported 33 CRS cases. In 1999, 2 cases were reported, but none were reported in 2000 and 2001.
Chile. In 1999, Chile implemented CRS surveillance. In total, 318 infants with suspected CRS have been investigated. Of those cases, 13 were confirmed as CRS: 4 occurred after the campaign, 3 in 1999, and 1 in February 2000. These mothers were infected before the campaign. In 2001, 452 suspected cases were reported and a blood sample was taken; however, no cases were confirmed as CRS.
Costa Rica. In 1981, a CRS compulsory notification registry was established. Since 1992, no CRS cases have been reported despite two outbreaks in 1993 and 1998–1999 during which a majority of cases were adolescents and adults. A review to identify infants <3 months old for CRS was conducted by using laboratory data that listed infants who had rubella IgM+ serology at the National Hospital for Children from January 1996 to 2000. In all, 49 infants were identified with rubella‐positive IgM. All infants were suspected of a TORCH infection (toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, varicella virus, syphilis); however, rubella was not shown as the causative agent in any of the cases. The initial presenting signs and symptoms were hepatosplenomegaly, 20 (41%); microcephaly, 10 (20%); multimalformation, 10 (20%); cataracts, 2 (4%); and no written recorded cause, 7 (15%). Because of the lack of detection of CRS cases, the Ministry of Health established a new CRS surveillance system.
Brazil. In 1996, the Ministry of Health included CRS in the mandatory notification list. Between 1997 and 2000, 876 suspected cases were reported, of which 132 were confirmed. Following the rubella outbreaks of 1998–2000, with a high incidence among young adults, 38 and 78 CRS cases were reported in 1999 and 2000, respectively.
Current Status of Rubella Vaccination Strategies
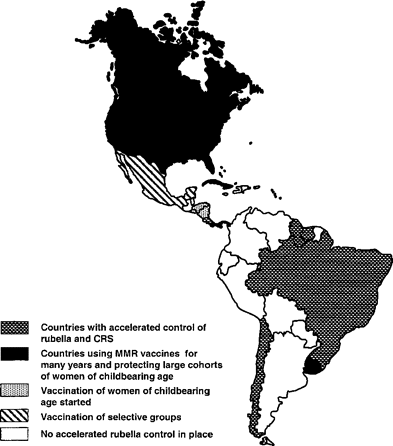
By 1986, 16 years after licensure of the rubella vaccine, 6 countries (the United States, Canada, Cuba, Panama, Costa Rica, and Uruguay) included MMR vaccine in their childhood programs. By October 2002, 41 countries and territories had rubella‐containing vaccine (MR or MMR) in their national childhood immunization programs. The remaining 3 countries, Peru, Haiti, and the Dominican Republic, plan to introduce a rubella‐containing vaccine in their national schedules starting in 2003 and 2004. In 1999, to reduce the risk of rubella infection in women of childbearing age, the English‐speaking Caribbean countries and Chile, Costa Rica, Panama, Brazil, and Honduras introduced routine vaccination at postpartum, after abortion, and in family planning clinics (figure 3).
Countries with accelerated rubella/congenital rubella syndrome (CRS) control programs in the Americas by strategy, June 2002. MMR, measles‐mumps‐rubella.
Mass Campaigns for Accelerated CRS Prevention or Rubella Elimination
Recently, several countries conducted adult mass campaigns for accelerated CRS prevention or rubella control. Brazil and Chile conducted campaigns for adult women only, while the English‐speaking Caribbean countries and Costa Rica had campaigns for adult men and women.
English‐speaking Caribbean. Between 1998 and 2001, 18 of 19 countries had adult rubella mass campaigns. Bermuda chose not to conduct a mass campaign because it routinely vaccinates women of childbearing age. The targeted population, men and women aged 20–39 years, was about 2.16 million. As of November 2001, over 75% of the target population of all countries had been vaccinated and coverage rates were 64%–97%. Countries that failed to achieve a national coverage of 80% conducted mop‐up campaigns. Since these successful campaigns, no CRS cases have been confirmed since 2000.
Chile. Between August and September 1999, the Chilean Ministry of Health conducted a mass vaccination campaign aimed at females aged 10–29 years (estimated target population, 2,507,448). The national coverage achieved was 98%. The lowest coverage (92%) was in women 20–24 years old. This success can be attributed in part to a high‐level social mobilization effort, and the efficiency, effectiveness, and commitment of health teams involved in the campaign.
Costa Rica. The Ministry of Health, in close coordination with the country’s social security system, implemented a strategy for accelerated control of rubella/CRS and measles. As part of this strategy, an adult mass campaign targeting men and women 15–39 years old was conducted in May 2001. The targeted population was 42% (1,606,329) of Costa Rica’s population. The campaign reported a national coverage above 95%. In seven regions, coverage was ⩾90% and in some it reached 100%. Vaccination coverage in 60 (75%) of the municipalities achieved coverage of ⩾95%; in the other 22 municipalities, coverage was 80%–95%. Rapid monitoring of coverage was useful to demonstrate that all provinces and municipalities reached the proposed target (⩾95%) and to identify reasons behind failure to vaccinate.
Brazil. To achieve the goal of accelerated CRS prevention, an adult female campaign was conducted in two phases, November 2001 and July 2002, and provided MR vaccinations to >28 million women. Each state established the target group and introduction date for the MMR vaccination and revaccination campaigns. The most common target age group was 12–39 years. On average, vaccination coverage reached 94% (range, 79%–99%) in the states. Municipalities that failed to reach coverage above 95% have continued mop‐up campaigns among unvaccinated groups that were identified through rapid monitoring of coverage. Women who were pregnant during the campaign were vaccinated immediately after they gave birth [15].
Cost Analysis
In the Americas, rubella vaccination programs have resulted in significant reduction of morbidity and mortality and in cost savings. The annual cost of treating an infant with CRS over a lifetime is estimated as $63,900 in Guyana, including indirect costs, and about $50,000 in Barbados. The Jamaica Ministry of Health estimates that the annual direct costs associated with caring for a child with CRS could be about $13,483 [8]. In the United States the lifetime cost of treating a patient with CRS is estimated at more than $200,000.
We estimate that even with the strategies now in place, 1500 CRS cases will occur over the next 15 years. Expenditures for rehabilitation and care of these cases, without counting the human suffering, are estimated at more than $60 million over 15 years. The implementation of the strategy to vaccinate men and women to interrupt rubella transmission and to prevent the occurrence of CRS over this period will cost about $4.5 million (7% of the total costs of care and rehabilitation of CRS cases). For the entire English‐speaking Caribbean, to interrupt the transmission of rubella and to prevent CRS, the cost‐benefit ratio is 13.3&rcolon;1. The cost‐effectiveness of the mass campaigns is estimated to average $2900 per case of CRS prevented. Two countries (Barbados and Guyana) estimated their costs for interruption of transmission. For Barbados, the cost‐benefit ratio was 4.7&rcolon;1 and for Guyana, 38.8&rcolon;1, with cost effectiveness of $1633 per CRS case prevented.
Discussion
The enhanced surveillance system for measles developed in 1996 by the Pan American Health Organization to support the regional goal of measles eradication has been instrumental in highlighting widespread circulation of rubella virus in the Americas. Rubella surveillance data show that epidemics occur every 4–7 years, similar to those reported by industrialized countries during the prevaccine era [16]. The estimated average age of those infected varies from 1–4 years to 5–9 years. Since the introduction of rubella‐containing vaccine, the transmission has shifted toward susceptible young adults.
The number of countries and territories reporting rubella morbidity has increased rapidly, allowing for improved analysis of rubella disease burden. The increased attention to rubella and CRS has also resulted in advances in the integration of rubella and measles surveillance systems in the region, which in turn has improved the sensitivity and specificity of rubella diagnosis. Despite these significant advances, further efforts are needed in refining rubella surveillance, given the mildness and short duration of the clinical signs and symptoms of rubella infection, which still represent a challenge for accurate diagnosis.
Countries that have adopted an accelerated rubella control strategy must maintain an effective surveillance system with the ability to detect rubella activity, to document the impact of the appropriate rubella vaccination strategy, and to follow and obtain knowledge of each confirmed case, rather than simply tracking the location where the virus is circulating. The surveillance of rash and fever is currently the most effective tool. As part of the surveillance efforts, all suspected rubella cases should be laboratory confirmed.
CRS is a serious public health problem but there are limited surveillance data on CRS incidence. Because of underreporting, we have only a partial view of real disease burden. CRS may be diagnosed by its classic triad of clinical signs: cataracts, heart disease, and deafness [17]; however, many infants have only one manifestation or may present earlier with neonatal signs. CRS surveillance is complex because the clinical presentations may vary during a child’s first year of life, making it difficult to detect most hearing impairments. Because of the diagnostic difficulties, additional tools that can enhance the identification of suspected CRS cases have been developed. These include the collaboration with regional systems such the Perinatal Information System (SIP 2000) of the Latin American Center for Perinatology and Human Development and the Congenital Malformation Latin‐American Collaborative Study. Information collected includes history of exposure to rubella, clinical illness during the mother’s pregnancy, vaccination status of the mother, laboratory confirmation of maternal rubella, any congenital malformations, and hepatosplenomegaly and purpura in newborns.
Laboratory confirmation of a CRS diagnosis is recommended. Rubella IgM is readily detected in the first 6 months of life, and rubella virus may be isolated from nasopharyngeal swab for 6–12 months after birth. However, few clinical cases of rubella are confirmed by laboratory testing and few virologic specimens have been submitted for molecular typing. As countries establish accelerated programs for control of rubella and CRS, confirmation programs must be strengthened. Molecular typing of virus isolates will enable us to understand the source and the propagation of rubella outbreaks and CRS cases and to determine rubella strain variations. As countries embark on accelerated rubella control, documenting the endemic strain in each country will assist in determining whether the case is imported. Even though a country has eliminated rubella, virus importation may occur and will only be stopped when all countries have similar vaccination efforts.
By using a combined strategy of vaccinating adult women and children with rubella‐containing vaccine, Cuba was the first country to eliminate rubella and CRS, with the last CRS case reported in 1989 and the last rubella case in 1995. This goal was largely achieved by means of two mass vaccination campaigns during 1985–1986 that initially targeted women aged 18–30 years, followed by children aged 1–14 years [16]. The Cuban experience led the way for other countries and regions to undertake mass campaigns in adults.
The rubella elimination initiative of English‐speaking Caribbean countries has provided vital information on the successful implementation of mass vaccination campaigns for men and women and on the cost‐benefit of immunizing against rubella infection. Cost benefit is a key issue as countries weigh the relative costs of including vaccination against women of childbearing age in the national immunization schedule. To date, the benefits of accelerated control vaccination far outweigh the costs associated with the treatment and rehabilitation of children with CRS.
The studies on rubella and CRS from Cuba and the English‐speaking Caribbean countries helped shape the accelerated control initiatives of Chile, Costa Rica, and Brazil. The latter countries are providing additional critical knowledge on rubella control and CRS prevention that will enable development elsewhere of successful and sustainable vaccination strategies of adults to reach vaccination coverage ⩾90%.
Experience is being gained in the mass vaccination of heterogeneous population groups that include men, women, and adolescents. In Costa Rica, for example, 42% of the population (1.6 million people), including men and women, were vaccinated within 1 month. The mass vaccination of 28 million women in Brazil against rubella is also providing important lessons on the vaccination of large population groups. All of these countries used MR vaccines, except for Chile, which used the rubella vaccine. As countries embark on the vaccination of women of childbearing age, health care providers should ensure that only non‐pregnant women are vaccinated and that women receive vaccination postpartum. The impact of the rubella vaccination strategies is already evident as seen in the rapid reduction of CRS morbidity in Cuba, the English‐speaking Caribbean, and Chile and in the rapid interruption of rubella virus transmission in Costa Rica.
The principal rationale for an accelerated vaccination strategy is to reduce the time needed to interrupt rubella virus circulation and to prevent CRS. Most of the countries described have implemented routine childhood rubella vaccination, and this strategy protects children during their first year of life. Nevertheless, this strategy will likely take more than 20 years to control CRS, as several cohorts of childbearing women will remain susceptible to rubella virus. Therefore, PAHO actively supports efforts to accelerate the control of rubella through the implementation of a one‐time mass adult vaccination campaign. The cornerstone of the rubella control strategy in the Americas lies in the combined vaccination of adult men and women coupled with rubella vaccine introduction into national childhood programs. This strategy will concurrently achieve rapid reduction of rubella virus circulation while preventing the shift of disease burden to susceptible young adults, particularly women of childbearing age, thereby reducing the incidence of CRS and its serious sequels.
Acknowledgments
The PAHO Division of Vaccine and Immunization acknowledges the efforts of health staff at all levels in the ministries of health in the Americas for the successful control of rubella and prevention of CRS. The valuable knowledge and experience being generated will benefit global immunization initiatives. We also thank the March of Dimes, the primary initiative supporter, and Jose Luis di Fabio for reviewing and commenting on this article.
References
Financial support: March of Dimes.